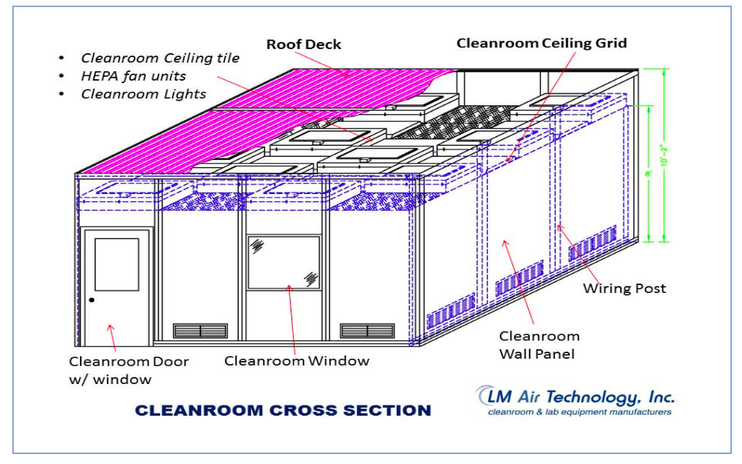
The construction and operation of cleanrooms is critical to many industries, particularly in the food, pharmaceutical and medical testing sectors. Upon completion of cleanroom construction, a series of validations and certifications must be performed to ensure compliance with relevant standards and regulations. In this paper, the completion of the clean room testing projects, acceptance criteria standards and industry certification requirements for detailed analysis and generalization.
I, the completion of the clean room testing program
1. Cleanliness:
Cleanliness is one of the core indicators of the clean room, through the detection of airborne particulate matter concentration to ensure that the clean room to meet the appropriate cleanliness level standards. According to iso 14644 standard, the cleanliness level of the clean room ranges from ISO 1 to ISO 10, and the lower the level, the less concentration of particles allowed. Commonly used cleanliness classes include
classes include:
iso 5: Suitable for environments that are extremely sensitive to particulate matter, such as semiconductor manufacturing and certain pharmaceutical processes.
iso 6: Suitable for most pharmaceutical and biologics manufacturing.
ISO 7: Commonly used in medical device and some food production.
iso 8: Suitable for general industrial use and non-critical production environments.
Cleanliness testing is usually performed using particle counters, which measure the number of particles in a given volume to ensure compliance with the relevant standards.
2. Temperature and Humidity
The control of temperature and humidity is critical to the operation of a cleanroom to ensure that it is within set limits to maintain product quality and operational safety. Conventional temperature control standards are usually: 18-22°C: this is the ideal temperature range for most laboratory and production environments.
3. Constant temperature and humidity
Required to maintain a temperature of 22 ± 1°C and humidity between 45-65%, this environment helps to reduce static electricity and improve operational comfort.
In some special cases, extreme humidity control is required, such as less than 40% relative humidity. Such environments usually utilize rotor dehumidification technology or highly efficient air conditioning systems to ensure stable environmental conditions.
4. Noise
Noise levels are assessed not only in relation to operator comfort, but also in relation to the proper functioning of equipment within the cleanroom. The noise level of the cleanroom should be in accordance with the relevant industry standards, usually requiring a noise level of less than 65 dB. Excessive noise may affect the operator's concentration and work efficiency, so the isolation of the noise source and acoustic design should be taken into account in the design and construction process.
5. Differential pressure
Differential pressure is another key parameter that monitors the pressure difference between the cleanroom and the outside environment to ensure the cleanroom's ability to prevent contamination. Proper differential pressure prevents external contaminants from entering the cleanroom. Common differential pressure requirements include: Positive pressure cleanrooms: typically require a higher internal pressure than the external environment to prevent contamination.
Negative pressure areas: In some cases, such as when handling hazardous substances, negative pressure is required to prevent contaminants from escaping.
6. Illumination
Illumination measurement to ensure that the lighting in the clean room to meet the operational needs of the common illumination standards: 300-1000 Lux: for different work areas, to ensure that the operator in the fine operation of sufficient light.
7. Antistatic
Anti-static measures assess the ability to control static electricity in a clean room, especially critical in the production of semiconductors and electronic equipment. Ensure the safety of the environment through the use of anti-static materials, grounding of floors, and regular monitoring of static voltage. 7. Airflow
Airflow is measured to ensure that the air flow in a clean room meets the design requirements, often ensuring that the air is exchanged at a frequency that maintains the cleanliness of the air. Common airflow requirements are: air exchange per hour: generally more than 20 times, depending on the level and use of the clean room.
II, the clean room acceptance norms and standards
Acceptance of the clean room needs to be based on relevant national and international standards. The main norms include:

1. China clean plant acceptance specification
Clean plant construction and quality acceptance specification GB51110-2015
Code for clean plant construction and quality acceptance +GB+51110-2015
Editor-in-chief: Ministry of Industry and Information Technology of the People's Republic of China
Approval Department: Ministry of Housing and Urban-Rural Development of the People's Republic of China
Implementation date: February 1, 2016
Announcement of the Ministry of Housing and Urban-Rural Development of the People's Republic of China
This is the basic standard for cleanroom construction and operation in China, covering cleanliness, environmental parameters and other requirements.
2. U.S. FDA cleanroom-related testing standards
cleanroom standards involving the pharmaceutical industry emphasize strict requirements for air quality and environmental control.
GMP specifications, EU, ISO, FDA on cleanliness monitoring requirements of the comparison of the U.S. FDA laboratory test quality management standard regulations and standards interpretation
III, the industry cleanroom-related certification
Obtaining industry-recognized certification is a necessary step in the operation of the cleanroom, different areas have different certification requirements:
1. food industry
- International: GMP certification (Good Manufacturing Practice)
- China: SC certification (food production license)
2. Medical devices
- International: GMP certification
- China: Medical Device Production License
3. Medical Operating Room
- International: QUAD A certification
- China: License to practice in medical institutions
These certifications are not only the recognition of the environmental requirements of the cleanroom, but also the guarantee of product quality and safety.
IV,Differential pressure monitoring of clean room
Differential pressure control of the clean room is an important means of maintaining its cleanliness and anti-pollution capabilities. The management of differential pressure can be realized in the following ways:

Control of air supply and exhaust air volume
When the air supply volume is greater than the air exhaust volume, positive pressure is formed in the clean room, and vice versa for negative pressure.
Use of control valves
The air volume of supply air and exhaust air is controlled by manual regulating valve to realize the regulation of differential pressure.
Automated Control System
In advanced cleanrooms, PLC control systems and differential pressure sensors are usually used to automatically adjust the air supply and exhaust volume by precisely controlling the opening angle of motorized valves to ACHieve precise control of differential pressure.
Verification and certification of a cleanroom is an important part of ensuring that it meets industry standards and safety requirements. Through rigorous as-built testing, adherence to acceptance specifications, and obtaining the necessary industry certifications, cleanrooms are able to fulfill their role in areas such as food, pharmaceutical, and medical testing. Understanding and mastering these steps will provide a solid foundation for effective cleanroom operations.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



