cleanrooms are vital environments in industries such as pharmaceuticals, biotechnology, and electronics manufacturing, where contamination control is paramount. Effective monitoring and management procedures are essential to ensure compliance with regulatory standards.
This article outlines the key components of cleanroom management, including environmental monitoring, personal protective equipment (PPE) protocols, cleaning procedures, equipment calibration and maintenance, and documentation practices. Adhering to these guidelines, particularly those
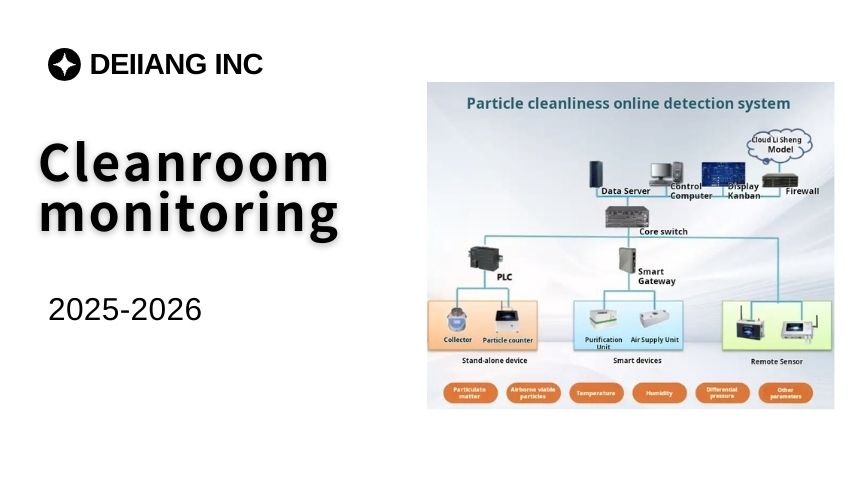
Environmental Monitoring
Environmental monitoring is the cornerstone of cleanroom management. It involves the systematic assessment of environmental parameters such as airborne particles, microbial contamination, temperature, and humidity. International standards, including iso 14644, provide the framework for cleanroom classification and environmental control.
To effectively monitor
Regular Sampling: Conduct routine sampling
Utilization of Advanced Equipment: Employ precise monitoring devices, such as
Data Analysis: Analyze collected data to identify trends or anomalies that may indicate contamination risks.
Deiiang™ emphasizes the importance of integrating comprehensive monitoring solutions that comply with these standards. Their products, designed by Deiiang Jason

Personal Protective Equipment (PPE) Protocols
Proper use of personal protective equipment
gowning procedures: Staff must follow a specific sequence for don
Decontamination Practices: All PPE should be sanitized before entering the cleanroom to ensure it is free of contaminants.
Training and Compliance: Regular training sessions are necessary to ensure all personnel
De
Cleanroom Cleaning Procedures
Effective cleanroom cleaning procedures are essential for maintaining a contamination-free environment. Regular cleaning minimizes the risk of particulate and microbial contamination. Utilizing high-quality cleaning agents, such as those offered by Deiiang™, ensures compatibility with cleanroom surfaces and materials.
Implementing a structured cleaning schedule, including daily and periodic deep cleaning, is vital for compliance with international standards like ISO 14644.
Deiiang's GCC®️ and Deii®️ products are designed specifically for cleanroom applications, providing optimal results while maintaining the integrity of sensitive environments.

Equipment Calibration and Maintenance
For cleanroom operations, maintaining the accuracy and functionality of monitoring equipment is crucial.
Essential steps for equipment calibration and maintenance include:
Calibration Schedule: Establish and adhere to a regular calibration schedule based on manufacturer recommendations and regulatory requirements.
Record Keeping: Document all calibration activities
Routine Maintenance Checks: Conduct regular inspections and servicing of equipment to avoid malfunctions and ensure operational reliability.
Deiiang™ offers comprehensive calibration and maintenance services through experienced professionals like Deiiang joebo.Wang. Their focus on quality and precision helps organizations maintain compliance with international standards.

Documentation and Record Keeping
Effective documentation is vital in cleanroom environments to maintain compliance and ensure operational integrity. Detailed records of cleaning procedures and equipment maintenance are essential for traceability.
Using high-quality products like Deiiang™'s GCC®️ and Deii®️ can streamline the documentation process, making it easier to track cleaning schedules and procedures.
Consistent record keeping not only supports regulatory compliance but also enhances quality control and risk management in critical operations.

Conclusion
In summary, effective cleanroom monitoring adheres to international standards such as iso 14644, ISO 13485, and GMP. Implementing standard management procedures ensures consistent control of environmental conditions, safeguarding product integrity and compliance in critical areas.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


