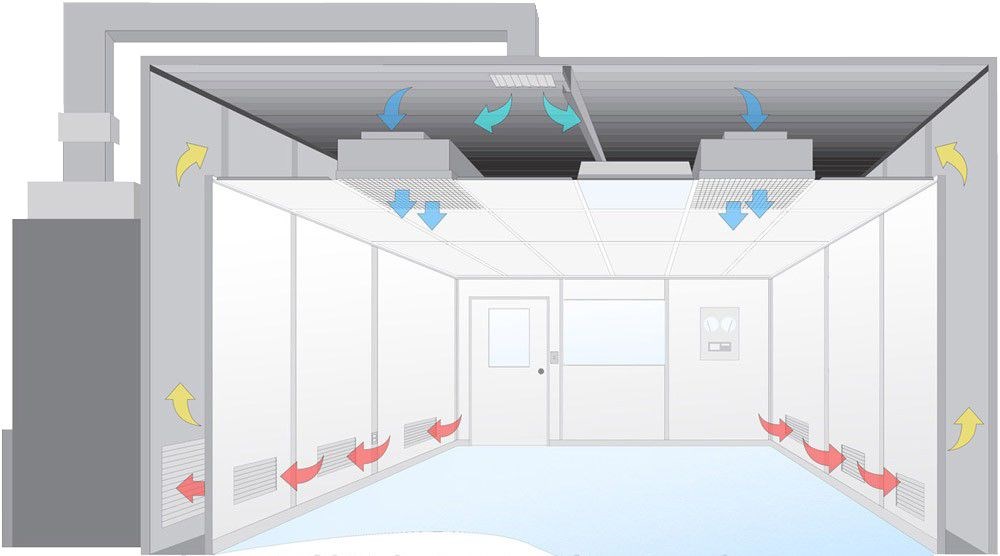
Types of Pharmaceutical Cleanrooms

Pharmaceutical cleanrooms are vital for maintaining the integrity of drug production processes. They are classified into various types based on cleanliness standards, operational requirements, and intended use. This article analyzes the primary types of pharmaceutical cleanrooms, including their specifications and regulatory compliance.
Class 100 Cleanrooms allow a maximum of 100 particles per cubic foot for particles ≥ 0.5 microns. This classification is essential for the manufacturing of sterile products, such as injectable drugs. According to ISO 14644-1, the cleanliness level is crucial in preventing contamination that could compromise product safety and efficacy.

Class 10,000 Cleanrooms
Class 10,000 cleanrooms permit up to 10,000 particles per cubic foot for particles ≥ 0.5 microns. These environments are often used for activities involving non-sterile products where the risk of contamination is moderate. Compliance with iso 14644-1 ensures that this level of cleanliness supports quality control in pharmaceutical manufacturing processes, particularly in the early stages of drug development.
Class 1,000 Cleanrooms
Class 1,000 cleanrooms can have up to 1,000 particles per cubic foot for particles ≥ 0.5 microns. This type is typically utilized for manufacturing pharmaceuticals that are sensitive to contamination but do not require a sterile environment. The guidelines outlined in ISO 14644-1 and the FDA’s cGMP regulations emphasize maintaining appropriate cleanliness levels to ensure product integrity and safety.

Type c CleanRooms
Type C Cleanrooms are defined by their specific cleaning protocols rather than strict particle count. They are often required in areas where non-sterile products are handled, necessitating daily cleaning and maintenance. Regulatory frameworks such as the European Medicines Agency (EMA) guidelines dictate that these environments must meet defined cleaning and personnel protocols to mitigate contamination risks.
Critical Zones and Transition Areas
Pharmaceutical cleanrooms often include critical zones and transition areas. Critical zones are where products are exposed to the environment, and they require the highest cleanliness standards. Transition areas act as buffer zones to minimize contamination during movement into and out of cleanrooms. The FDA and ISO guidelines recommend stringent controls in these areas to ensure compliance with cleanliness requirements.
Type A Cleaning in Pharma

Definition of Type A Cleaning
Type A cleaning refers to areas where non-sterile products are handled. It focuses on maintaining cleanliness through regular cleaning protocols and controlled access to minimize contamination risks during pharmaceutical manufacturing.
Cleaning Protocols
In Type A environments, strict cleaning protocols are enforced. Personnel must follow specific procedures, including the use of appropriate cleaning agents and disinfectants to ensure surfaces remain free from contaminants.
Type B Cleaning in Pharma

Definition of Type B Cleaning
Type B cleaning environments are designated for handling sterile products. They require higher cleanliness standards and stricter controls to prevent any contamination during the production of pharmaceuticals.
Airflow and Filtration
Type b Cleanrooms utilize advanced airflow systems with high-efficiency particulate air (HEPA) filters. This ensures a constant flow of filtered air, minimizing the risk of airborne contaminants in sterile areas.
Validation and Monitoring
Type b CleanRooms require continuous monitoring and validation. Regular environmental testing and data logging systems are essential to ensure compliance with regulatory standards and maintain air quality.
The Difference Between Grade B and Grade C Cleanroom

Usage Applications
Grade B is typically used for aseptic manufacturing processes, where sterility is critical. Grade C cleanrooms are suitable for supporting operations that require less stringent cleanliness, such as certain filling processes.
Operational Controls
Operations in Grade B cleanrooms are subject to stricter controls, including more frequent monitoring and stringent gowning procedures. Grade C environments have more relaxed protocols but still require adherence to good manufacturing practices.
Class D Air Quality

1. Definition of Class D Air Quality
Class D air quality refers to environments where the maximum allowable particle count is 3,520,000 particles per cubic meter for particles ≥ 0.5 microns. This classification is suitable for less critical pharmaceutical processes.
2. Applications of Class D
Class D environments are often used in areas like packaging or storage of non-sterile pharmaceuticals. While they do not require the stringent controls of higher-grade cleanrooms, maintaining a reasonable cleanliness level is still essential.
3. Operational Considerations
In Class d Cleanrooms, operational practices may include regular cleaning and monitoring of surfaces. Although less rigorous than higher-class environments, procedures should still prioritize contamination control to protect product integrity.
What is a Class C Clean Air Zone?

1. Usage Scenarios:Class C clean air zones are typically used in processes such as formulation and secondary packaging. These areas balance cleanliness with operational efficiency, making them suitable for certain pharmaceutical applications.
2. Cleaning and Maintenance:Regular cleaning procedures are essential in Class C zones to manage contamination risks. Personnel must follow established protocols to maintain cleanliness while ensuring efficient production processes.
3. Compliance and Monitoring:Class C clean air zones require ongoing monitoring to ensure compliance with industry standards. Environmental monitoring systems help track particle levels and maintain the required air quality for effective manufacturing operations.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU