What Are the Cleanroom Classifications?
Cleanroom classifications are a standardized way of defining the cleanliness level of a controlled environment. These classifications are based on the concentration of airborne particles within the cleanroom.
Clean environments are essential for industries like pharmaceuticals, biotechnology, electronics, aerospace, and food production, where contamination could compromise product quality or safety.The most widely used standard for cleanroom classification is iso 14644-1, which defines cleanroom classes from ISO 1 (the cleanest) to ISO 9 (the least clean).
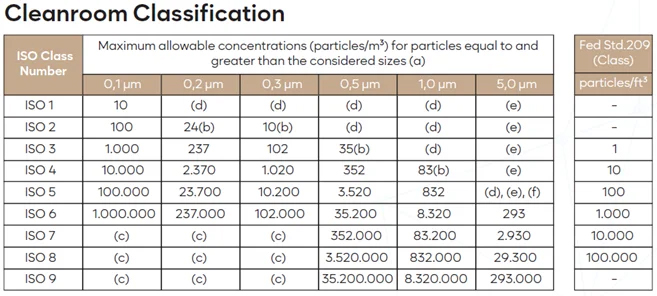
Each class has specific limits on the maximum number of airborne particles of different sizes that are allowed per cubic meter of air.
ISO Cleanroom Classifications
Below is a summary of the particle limits for each ISO cleanroom class:
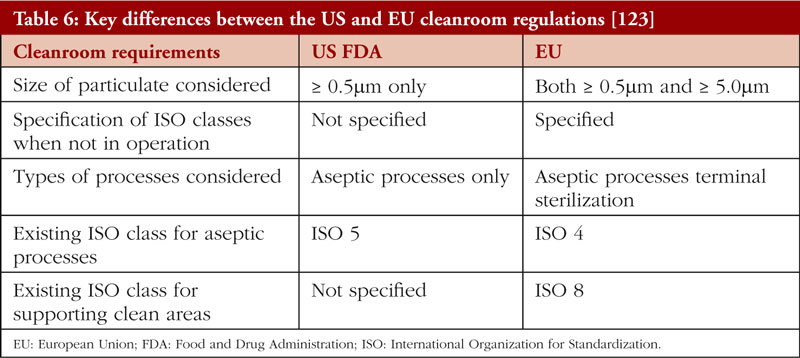
Key Features of Cleanroom Classifications
1. Particle Limits
Each classification sets strict limits for particles of different sizes to control contamination. ISO 1 has the strictest limits, while ISO 9 allows higher particle concentrations.
2. Applications
The required cleanliness level depends on the industry and the sensitivity of the processes or products being handled. For instance, iso 5 is commonly used for sterile pharmaceutical production, while iso 8 is suitable for general manufacturing.

3. Additional Controls
Filtration: Cleanrooms are equipped with HEPA or ULPA filters to remove airborne particles.
Airflow and Pressure:Proper airflow patterns and positive pressure ensure contaminants are kept out of the cleanroom.
Environmental Factors:Temperature and humidity are often controlled to maintain optimal conditions for sensitive processes.
What Are the gmp cleanliness Classifications?
Good Manufacturing Practice (GMP) cleanliness classifications refer to the standards and guidelines set to ensure a controlled environment in industries such as pharmaceuticals, biotechnology, and food production.
GMP cleanliness classifications are designed to minimize the risk of contamination in the manufacturing and handling of products that are sensitive to contaminants, such as drugs, medical devices, and food.
These classifications are similar to cleanroom classifications but are specifically tailored to meet the requirements for product safety, quality, and compliance with regulatory standards.
The classifications are typically aligned with ISO 14644-1 cleanroom standards, with specific guidelines for particle limits, air quality, and contamination control measures.
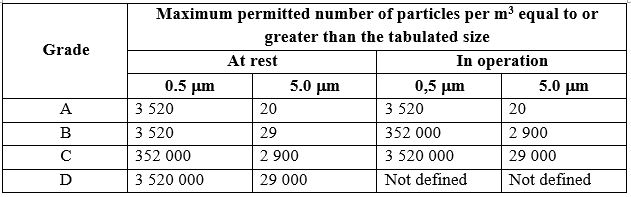
What is GLP Class A and B Cleanroom Environments?

1. GLP Class A cleanroom environment
GLP Class A cleanrooms are the highest level of controlled environments in laboratory settings. They are designed for the most sensitive processes that require the strictest contamination control to prevent even the smallest amounts of airborne particles or microorganisms from affecting the results. Key features of Class A cleanrooms include:
Strict Particle Control: class a environments have extremely low particle concentrations, typically allowing fewer than 10 particles per cubic meter of air (0.3 microns or larger).
High-Efficiency Air Filtration: These cleanrooms use HEPA or ULPA (Ultra-Low Penetration Air) filters, which are highly effective in removing airborne particles. The filtration system ensures that particles as small as 0.3 microns are captured.
Aseptic Conditions: GLP Class A cleanrooms are critical for processes that involve the direct exposure of sterile products or substances. This includes tasks such as sterile filling, preparation of aseptic solutions, or work with sensitive biological or chemical substances.
2. GLP class b cleanroom Environment

GLP Class B cleanrooms, while still highly controlled, are less stringent than Class A environments. They are designed for processes that require high levels of cleanliness but do not involve direct exposure of the product to the environment. Some of the key characteristics of Class B cleanrooms include:
Moderate Particle Limits: Class B environments allow a higher concentration of airborne particles than Class A. Particle limits may be in the range of 100 to 1,000 particles per cubic meter for particles 0.5 microns or larger, depending on the specific requirements.
Air Filtration: Class B cleanrooms use HEPA filters to ensure that airborne particles are kept at acceptable levels, though the filtration is not as stringent as in Class A environments.
Controlled but Less Critical: Class B cleanrooms are commonly used for processes such as storage or non-sterile preparation of products. They are suitable for tasks like material handling, compounding, or areas where aseptic processing is conducted but not in direct contact with the final product.
3. Applications of GLP Class A and B Cleanrooms

Class A Applications: These cleanrooms are crucial for processes that require the highest level of contamination control, such as sterile manufacturing, aseptic filling, and handling of sensitive materials in pharmaceuticals, biotechnology, and medical device industries.
Class B Applications: While still controlled, these cleanrooms are used for processes such as non-sterile product preparation, material storage, and work involving less sensitive products that do not require the same level of contamination control as Class A environments.
What are the FDA classifications?
The FDA (Food and Drug Administration) classifies medical devices and other regulated products into categories based on the level of risk they pose to patients and users.
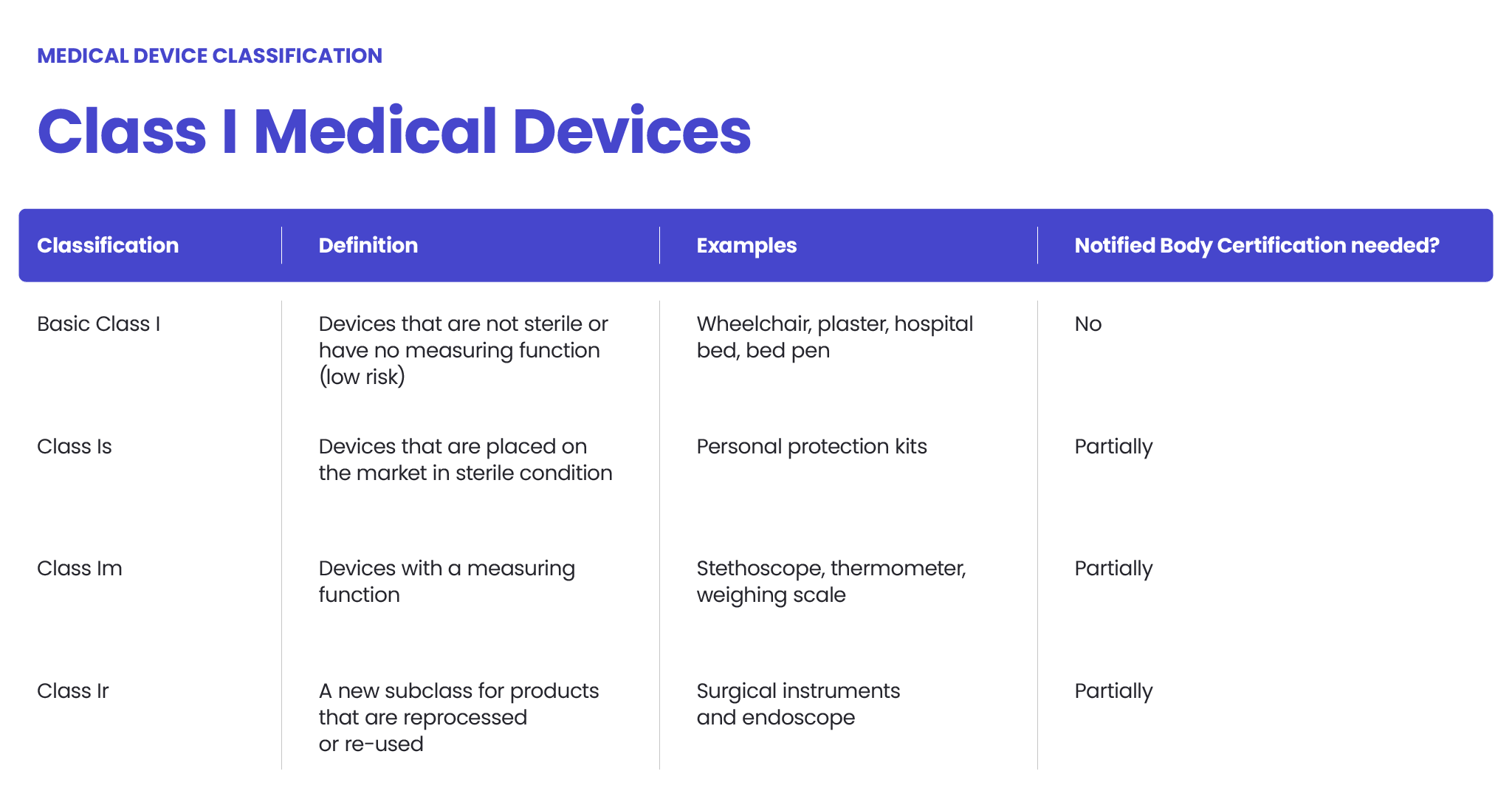
This classification system helps determine the regulatory requirements for each product, including the level of scrutiny, safety, and efficacy evidence required for approval. The FDA uses a three-tier system—Class I, Class II, and Class III—each with different regulatory controls.
These classes ensure that products are safe and effective before they reach the market, protecting public health.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



