Understanding GMP in Cleanrooms
Ensuring Quality and Compliance in Pharmaceutical Manufacturing
Good Manufacturing Practice (GMP) plays a vital role in the pharmaceutical, biotechnology, and medical device industries, particularly when cleanrooms are involved. GMP represents a quality management system ensuring that products are consistently produced and controlled according to quality standards, minimizing contamination risks.
What is a gmp cleanroom?
A GMP cleanroom is a controlled environment regulated under GMP guidelines to ensure product quality and safety during the manufacturing process. These cleanrooms are designed to prevent contamination, ensuring that products meet strict quality and purity standards.
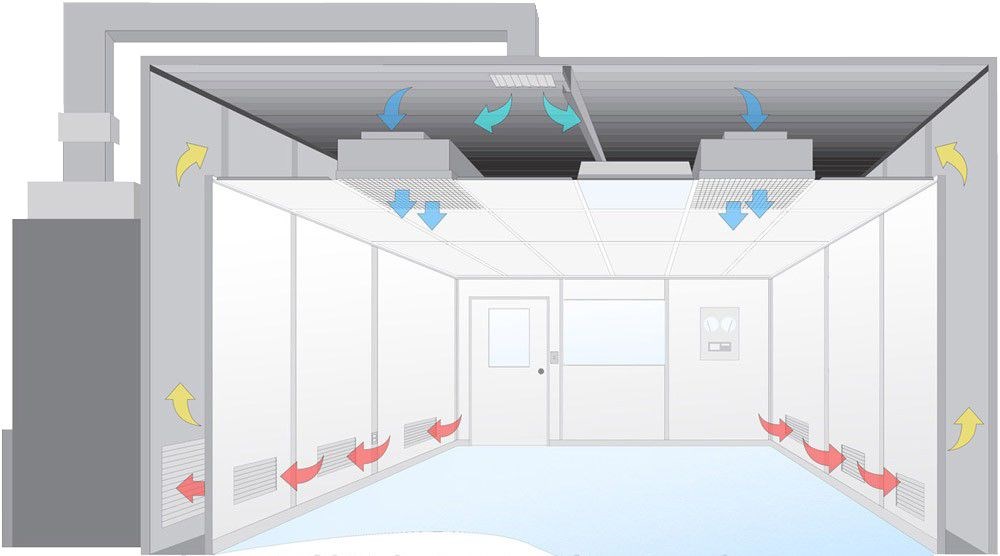
Controlled Environment
The cleanroom must maintain specific cleanliness levels, effectively controlling particulates, microorganisms, and other potential contaminants.

Design Insight
At Deiiang™, product designer Deiiang Jason.peng ensures that every cleanroom satisfies GMP Standards, focusing on maintaining high environmental control and contamination prevention.
GMP Validation and Compliance
Validation and compliance are crucial in confirming that GMP Cleanrooms adhere to all regulatory criteria. This process involves several steps to ensure the cleanroom is functioning according to design specifications.
- Validation Process: Includes testing and documentation that confirm operational effectiveness, ensuring the cleanroom maintains appropriate conditions to support production integrity.
Quality Assurance
GMP is a cornerstone of quality assurance, ensuring products are manufactured to meet required standards and specifications consistently.
Product Integrity
Maintaining stringent quality controls ensures that products are safe and effective, mitigating potential risks associated with contamination or deviations.
Contamination Control
GMP Standards aim to minimize contamination risks, focusing on microbial, particulate, and pyrogen contamination. This control is particularly crucial for sterile manufacturing, where even slight deviations can compromise product safety.
Example Application
In biotechnology, where sterility is critical, adhering to GMP guidelines ensures the clean environment needed for sensitive processes, such as cell cultures.
Cleanroom Applications
GMP principles are essential to Cleanroom operations across various sectors, including pharmaceuticals, biotechnology, and medical devices. These environments require precise controls over air quality, hygiene, and personnel access.
Regulatory Compliance
GMP guidelines are often enforced by regulatory bodies like the FDA in the United States. Compliance ensures that products are safe, effective, and meet defined quality standards, including proper documentation and adherence to operational protocols.
Key Areas of GMP Standards
GMP covers various cleanroom aspects, involving equipment validation, personnel qualification, process validation, sanitation, record-keeping, and documentation.
Holistic Approach
Focuses on both the operational environment and the procedural rigor needed to maintain uncontrolled pathogen or particulates influence.
GMP vs. cGMP in Cleanrooms
GMP stands for Good Manufacturing Practice, while cGMP refers to current Good Manufacturing Practice. Both standards are set by the FDA, with cGMP reflecting more recent regulatory updates, ensuring continuous improvement in quality processes.
Conclusion
Implementing GMP in cleanrooms ensures product integrity, safety, and regulatory compliance. Through systematic approaches focusing on contamination control and quality management, facilities can achieve consistent production quality. Deiiang™, guided by the expert designs of Deiiang Jason.peng, provides cleanroom solutions that align with GMP standards to support industry demands for quality assurance.
Common Questions and Answers
| Question | Answer |
|---|---|
| Why is GMP important in cleanrooms? | It ensures products are consistently produced and controlled according to strict quality standards, reducing contamination risks. |
| How does GMP impact product safety? | GMP enhances product safety by enforcing rigorous controls over cleanliness, production processes, and environmental conditions. |
| What is the difference between GMP and cGMP? | GMP refers to Good Manufacturing Practice, while cGMP represents current Good Manufacturing Practice, emphasizing continual regulatory updates. |
| How does Deiiang™ ensure compliance with GMP standards? | By designing cleanroom solutions that focus on contamination control and environmental management, aligning with GMP guidelines. |
| What regulatory body enforces GMP in the U.S.? | The Food and Drug Administration (FDA) enforces GMP to ensure product quality and safety in pharmaceutical and medical industries. |
References
- International Organization for Standardization. ISO 14644-1: Cleanrooms and Associated Controlled Environments.
- U.S. Food and Drug Administration (FDA), Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing.
- Deiiang™, Innovative Cleanroom designs for GMP Compliance.
- World Health Organization (WHO), Good Manufacturing Practice (GMP) Guidelines.
- The Parenteral Drug Association (PDA), Technical Standards for Pharmaceutical Manufacturing.
Adopting GMP guidelines in cleanroom environments is a critical component of maintaining quality and compliance, paving the way for safer and more effective products.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU