gmp class A and B Cleanroom Environments
Specialized Environments for Pharmaceutical Manufacturing
Introduction to gmp cleanrooms
GMP (Good Manufacturing Practice) Class A and class b cleanrooms are specialized environments designed to ensure the highest level of cleanliness and control during the manufacturing of pharmaceutical products, particularly sterile drugs and medical devices. These environments follow strict guidelines to prevent contamination, ensuring that products are produced safely and comply with regulatory requirements.
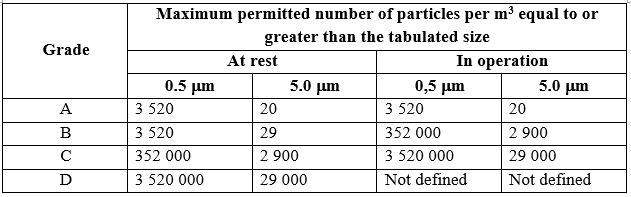
Cleanroom Classifications according to EU GMP
gmp class a CleanRoom Environment
A GMP Class A CleanRoom represents the most stringent standards for air cleanliness. This environment is typically used for processes that directly affect the sterility of pharmaceutical products, such as aseptic filling and sterile drug preparation.
According to the EU GMP Annex 1 and ISO 14644-1, Class A modular cleanrooms must meet iso 5 standards, meaning that the allowable particle concentration of 0.5 microns or larger is restricted to ≤3,520 particles per cubic meter.
gmp class b CleanRoom Environment
A GMP Class B cleanroom is slightly less stringent than Class A but still requires a high level of cleanliness. Class B environments are used for processes that support aseptic filling or other critical pharmaceutical processes but do not directly come into contact with the sterile products.
According to EU GMP Annex 1, these rooms must meet ISO 6 standards for particle count, which allows up to 35,200 particles per cubic meter for particles of 0.5 microns or larger.
Key Differences Between GMP Class A and Class B Cleanrooms
| Feature | GMP Class A | GMP Class B |
|---|---|---|
| Particle Count (0.5 microns or larger) | ≤3,520 particles/m³ (ISO 5) | ≤35,200 particles/m³ (ISO 6) |
| Primary Usage | Critical processes (aseptic filling, sterile preparation) | Supporting processes, buffer zones |
| Airflow System | Unidirectional airflow | Turbulent airflow with higher exchange rates |
| Filtration Efficiency | Higher efficiency (HEPA/ULPA) | Standard HEPA/ULPA filtration |
Features and Control Measures
Both GMP Class A and Class B cleanrooms are equipped with advanced air filtration systems, including HEPA or ULPA filters, to ensure the removal of airborne particles. Airflow patterns and pressure differentials are closely monitored to maintain cleanliness, and strict gowning and hygiene protocols are followed to minimize human contamination.
Filtration
Both classes use HEPA or ULPA filters,
but Class A typically requires higher filtration efficiency.
Airflow
Class A uses unidirectional airflow,
while Class B may use turbulent modular clean room airflow with higher air exchange rates.
Pressure
Both Class A and B require positive pressure,
to prevent contamination from surrounding environments.
Applications of GMP Class A and B Cleanrooms
Class A and Class B Cleanrooms are found primarily in the pharmaceutical and biotechnology industries, where the production of sterile products is required. Class A is used for the most sensitive processes, while Class B is used in secondary processes that support or are adjacent to Class A.

• Class A Applications
- Sterile drug manufacturing
- Aseptic filling processes
- Sterile preparation areas
- Direct contact with sterile products
- Critical aseptic operations
• Class B Applications
- Clean areas supporting Class A
- Compounding and preparation areas
- Buffer zones between Class A and lower grades
- Non-critical sterile support processes
- Equipment preparation for Class A areas
Relevant Standards and Guidelines
EU GMP Annex 1
Provides specific requirements for cleanroom environments in the pharmaceutical industry, outlining the necessary classifications for aseptic manufacturing.
ISO 14644-1
Defines CleanRoom Classifications, including ISO 5 and ISO 6, which align with GMP Class A and Class B standards.
ISO 14644-2
Establishes guidelines for monitoring and testing cleanrooms to ensure they meet the required standards.
FDA 21 CFR Part 211
U.S. regulations for the manufacturing of drugs, including requirements related to cleanroom environments.
GMP cleanroom engineering Services | Ensuring Pharmaceutical Manufacturing Excellence
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



