class d room Classification

1. Definition of Class D Room: A Class D room is a controlled environment categorized as iso8 according to ISO 14644-1. This classification is essential in pharmaceutical and biotechnology industries for areas with moderate cleanliness requirements, allowing a maximum of 3,520,000 particles per cubic meter for particles ≥0.5 micrometers.
2. Importance of Class D Rooms: Class D rooms play a crucial role in processes that require a controlled environment, such as compounding non-sterile pharmaceuticals. Maintaining cleanliness in these areas reduces contamination risks, ensuring product quality and safety for end-users.
3. Cleaning and Maintenance Protocols: Cleaning protocols in Class D rooms must be defined and documented according to Good Manufacturing Practices (GMP). It typically includes daily cleaning of surfaces with validated agents to ensure effective removal of contaminants and adherence to established cleanliness standards.
4. Monitoring and Validation: Regular environmental monitoring is critical in Class D rooms. This includes active air sampling, surface sampling, and monitoring of temperature and humidity. Validation of cleaning procedures is also essential to ensure consistent performance and compliance with regulatory standards.

Relevant Standards and Guidelines
ISO 14644-1:Cleanrooms and associated controlled environments – Part 1: Classification of air cleanliness. This standard defines the classification of cleanrooms based on the number and size of airborne particles.
iso 14698-1:Biocontamination control – Part 1: General principles and methods. This standard addresses the control of microbiological contamination in controlled environments, crucial for Class D rooms used in pharmaceutical applications.
FDA Guidance for Industry:This guidance document provides recommendations for maintaining cleanroom standards in pharmaceutical manufacturing, emphasizing the importance of environmental monitoring and personnel training.
EU GMP Guidelines:These guidelines provide regulations for Good Manufacturing Practices in the European Union, outlining requirements for cleanrooms and controlled environments, including Class D areas.
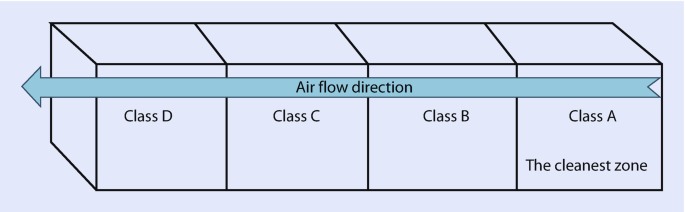
Class D in Clean Room

Definition of Class D
class d cleanrooms are classified as ISO8 environments, which allow a maximum of 3,520,000 particles per cubic meter for particles equal to or greater than 0.5 micrometers.
Applications
These cleanrooms are commonly used in pharmaceutical manufacturing, biotechnology, and electronics industries, where moderate cleanliness levels are essential for product quality and process reliability.
Air Quality Standards
In Class D cleanrooms, maintaining air quality is critical. The standard requires regular monitoring of particulate contamination to ensure compliance with established cleanliness levels.
The Requirements for grade d cleanroom Gowning

1. Required Garments
Personnel must don coveralls or gowns, gloves, masks, and hair covers before entering the cleanroom. These items help reduce the risk of contamination from human sources.
2. Footwear Requirements
Cleanroom-safe footwear, such as shoe covers or dedicated cleanroom shoes, is mandatory. This practice prevents dirt and particles from being tracked into the clean environment.
3. gowning procedures
Proper gowning procedures are essential. Personnel must follow a designated sequence when donning garments to ensure maximum protection and cleanliness upon entering the cleanroom.
What is gmp class A and B Cleanroom Environments?

Definition of Class A
Class A cleanrooms are classified as iso5 environments, requiring stringent controls on airborne particulate contamination. They are essential for aseptic processing and sterile product manufacturing.
Definition of Class B
class b cleanrooms are classified as ISO7 environments. They maintain lower particulate levels than Class A, serving as support areas for less critical operations.

Air Quality Control in Class A
Class A cleanrooms require a high level of air filtration and airflow control. HEPA filters are essential to maintain low particle counts and ensure a sterile environment during critical processes.
Air Quality Control in Class B
Class B cleanrooms also utilize HEPA filtration but allow for a higher particle count than Class A. They serve to support Class A environments by ensuring cleanliness during material preparation and equipment sterilization.
The FDA Classification of Clean Rooms

Classifications Explained
The FDA uses classifications such as Class A, B, C, and D to define cleanroom environments. Each class has specific requirements for airborne particulate levels and must adhere to stringent guidelines.
Compliance and Enforcement
The FDA enforces cleanroom classifications through inspections and regulatory audits. Facilities must demonstrate compliance with established cleanliness standards to maintain their license and ensure product integrity.
Documentation and Record-Keeping
Proper documentation and record-keeping are essential in FDA-regulated cleanrooms. These records provide evidence of compliance with cleanliness standards and facilitate traceability in case of contamination events.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



