Class A:
High-risk operation areas in pharmaceutical factories, such as filling areas, areas where rubber stopper barrels, open ampoules, open vials, and areas where aseptic assembly or connection operations are performed. Laminar flow operating tables (hoods) are usually used to maintain the environmental conditions of this area. The laminar flow system must evenly supply air in its working area, with a wind speed of 0.36-0.54m/s (guideline value). Data should be available to prove the state of laminar flow and must be verified.
In a closed isolation operator or glove box, unidirectional flow or lower wind speed can be used
Class B:
Refers to the background area where high-risk operations such as aseptic preparation and filling are located in the Class A area
Class C and Class D:
Refers to clean operation areas with lower importance in the production of sterile drugs.
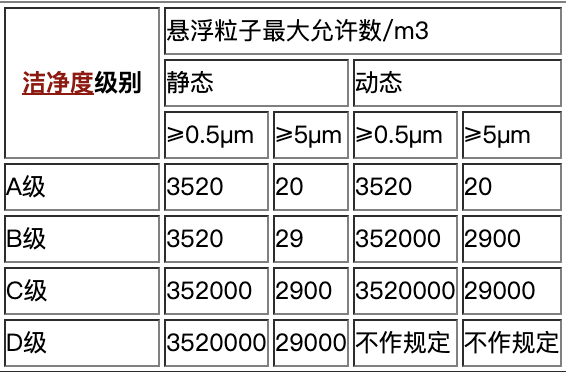
Notes:
A-level is 100-level, the background environment of A is higher and the requirements are stricter
B-level is 10,000-level;
C-level is equivalent to 100,000-level;
D-level is equivalent to 300,000-level;
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


