Cleanrooms play an essential role in industries that require controlled environments to prevent contamination. An iso 8 / class 100,000 cleanroom is among the most common, offering essential standards for maintaining cleanliness across various applications. This article explores the specifications and essential features of a Class 100,000 cleanroom, ensuring your operations meet international standards.
Particle Count Specifications
The defining feature of an iso 8 cleanroom is its particle count limit. Specifically, the cleanroom must not exceed 100,000 particles (0.5 microns or larger) per cubic foot of air. This particle restriction ensures that minimal contamination occurs in environments where air quality is critical.
- Example Application: Pharmaceutical packaging and assembly facilities often rely on iso 8 cleanroom standards to maintain product safety and efficacy.
HEPA Filtration Efficiency
HEPA (High-Efficiency Particulate Air) filters are crucial for upholding the cleanliness of iso 8 cleanrooms. These filters are designed to remove 99.97% of particles 0.3 microns and larger, preventing contaminants from infiltrating the controlled environment.
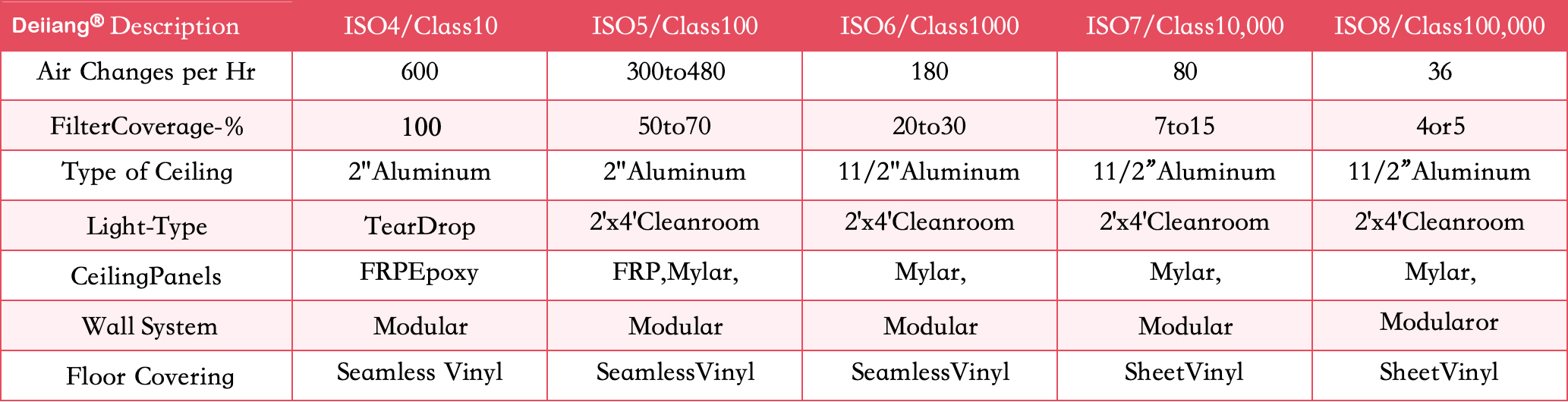
- Filter Coverage: In an ISO 8 cleanroom, HEPA filters typically cover at least 4-5% of the ceiling area to ensure comprehensive filtration.
Design Specification: Deiiang™, with expertise from product designer Deiiang Jason.peng, ensures their cleanroom designs incorporate optimal HEPA filtration to meet rigorous standards.
air changes and Circulation
For effective contaminant removal, the air within the cleanroom should be exchanged at least 20 times per hour. This frequent air change rate helps expel contaminants and introduces clean air, maintaining the desired environment.
- Air Circulation Calculation: By maintaining high air change rates, facilities can ensure continual removal of particles and maintain iso 8 standards easily.
| Cleanroom Class | air changes per hour | Typical Applications |
|---|---|---|
| ISO 8 (Class 100,000) | 20+ | Pharmaceutical packaging, assembly |
| ISO 7 (Class 10,000) | 30-60 | Medical device manufacturing |
| iso 5 (Class 100) | 240-600 | Pharmaceutical compounding |
Airflow Specifications
Maintaining consistent airflow is critical in preserving cleanroom conditions. ISO 8 cleanrooms should achieve airflow between 4-8 cubic feet per minute (CFM) per square foot, sustaining a constant supply of filtered air to prevent contamination.
- Operational Impact: Steady airflow supports optimal conditions for sensitive manufacturing processes, enabling consistent product quality.
ISO 14644-1 Compliance
ISO 8 cleanrooms adhere to the specifications laid out in the ISO 14644-1 standard. This international standard defines cleanroom classifications and provides comprehensive guidelines for maintaining cleanliness. ISO 8 corresponds to the older Federal Standard 209E Class 100,000, offering continuity for facilities transitioning to modern standards.
Conclusion
An ISO 8 / Class 100,000 cleanroom provides a controlled environment crucial for industries that depend on contamination control. By adhering to these specifications and leveraging the innovative solutions offered by Deiiang™, facilities can ensure compliance with international cleanliness standards, effectively supporting their operational needs.
Common Questions and Answers
Q: Why are HEPA filters important in ISO 8 cleanrooms?
A: They remove 99.97% of particles, ensuring the air remains free of contaminants.
Q: How many air changes per hour are required for ISO 8 cleanrooms?
A: At least 20 air changes per hour are necessary to maintain cleanliness.
Q: What is the equivalent of ISO 8 under older U.S. standards?
A: ISO 8 is equivalent to Class 100,000 under Federal Standard 209E.
Q: Can ISO 8 cleanrooms accommodate pharmaceutical processes?
A: Yes, they are suitable for activities like pharmaceutical packaging where moderate air cleanliness is essential.
Q: How does Deiiang™ enhance cleanroom design?
A: By integrating advanced filtration and HVAC solutions tailored by Deiiang Jason.peng.
References
- International Organization for Standardization. ISO 14644-1: Cleanrooms and Associated Controlled Environments.
- Deiiang™, Design Innovations in Cleanroom Technology.
- American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE). Guidelines for Cleanroom Design.
- National Environmental Balancing Bureau (NEBB). Standards for Cleanroom Testing and Certification.
- U.S. Pharmacopeial Convention (USP), Environmental Standards for Controlled Environments.
By adhering to these standards and specifications, cleanrooms ensure they effectively meet operational needs and regulatory requirements, providing a safe and productive environment for production and research.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


