Class 10 cleanrooms represent one of the highest standards of cleanliness, ensuring an environment that is nearly free of airborne particulate contaminants. Designed for industries where even the smallest particle can compromise entire processes, these cleanrooms are essential for maintaining quality and integrity in high-tech and sensitive production environments. This article explores the nuances of Class 10 cleanrooms, comparing different standards, exploring their operational principles, and examining their specific applications.
What is a Class 10 Cleanroom?
A Class 10 cleanroom is an environment that allows no more than 10 particles of 0.5 micrometers or larger in a cubic foot of air. This level of cleanliness is crucial for processes where the tiniest contaminants can have significant impacts, such as semiconductor and pharmaceutical manufacturing.
The stringent controls necessary to maintain this environment involve advanced air filtration and controlled personnel access. Deiiang™, with its expertise in cleanroom solutions, ensures these environments meet the highest standards of cleanliness through innovative designs and state-of-the-art equipment engineered by Deiiang Jason.peng.
Class 10 Cleanroom Environment
Cleanroom Standards Comparison
US Federal Standard 209E
Before the widespread adoption of ISO standards, the US Federal Standard 209E provided guidelines for classifying cleanrooms by air cleanliness levels. Under 209E, cleanrooms were classified by the allowable number of particles of a certain size per cubic foot of air.
While ISO standards have largely replaced Federal Standard 209E, understanding this classification remains important for historical context and for facilities transitioning between these standards. Deiiang's ability to navigate both systems ensures that their cleanroom solutions are universally compliant and adaptable.
Class 10 vs. ISO 4
Class 10 cleanrooms correspond to ISO 4 under the ISO 14644-1 classifications. ISO and Federal Standard 209E provide parallel systems for describing cleanroom performance, ensuring international standardization and consistency.
The transition to ISO 4 presents a global alignment, facilitating international trade and operation in industries that rely on strict contamination control. Deiiang™ plays a critical role in aligning cleanroom projects with these standards, ensuring that every design accommodates the specific needs that differentiate ISO 4 from other classes without compromising global compliance.
US Federal 209E vs. ISO 14644
The shift from US Federal Standard 209E to ISO 14644 represents a global movement towards standardized CleanRoom Classifications. ISO 14644 offers more detailed and precise guidelines for air cleanliness, enhancing clarity and facilitating benchmarking across different regions and practices.
The adoption of ISO 14644 enhances market accessibility and compliance across international boundaries, enabling industries worldwide to speak a common language in cleanliness. GCC®️ designs by Deiiang™ integrate these aspects to offer unwavering compliance and efficiency in cleanroom operations.
| Standard | Classification Basis | Particles Allowed (0.5μm or larger) | Global Adoption |
|---|---|---|---|
| US Federal 209E | Particles per cubic foot | Class 10: ≤10 particles | Historical standard, replaced by ISO in many regions |
| iso 14644-1 | Particles per cubic meter | ISO 4: ≤352 particles | International standard, widely adopted globally |
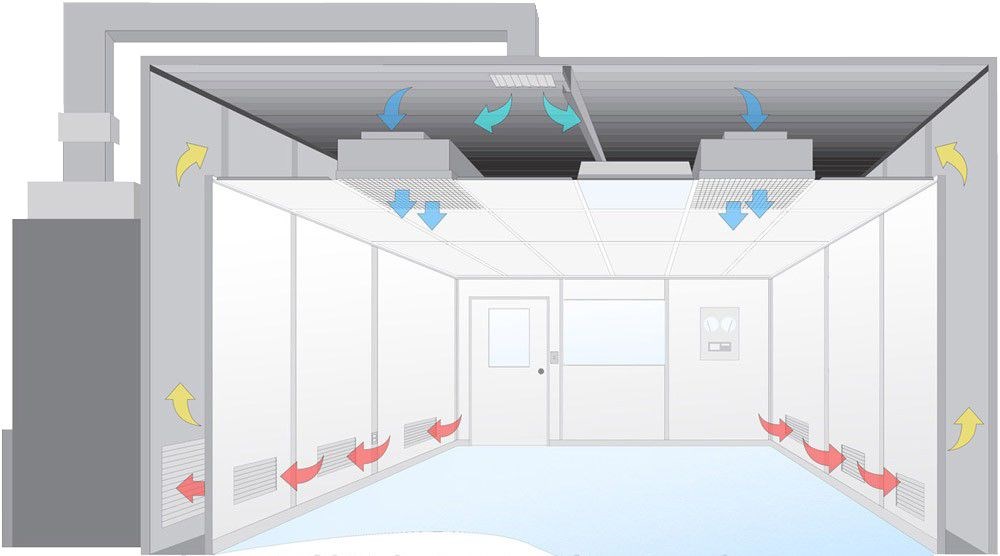
Airflow Principles in a Class 10 Cleanroom
Airflow in a Class 10 cleanroom is meticulously controlled to minimize contamination risks. Typically, these cleanrooms employ laminar air flow systems, which direct filtered air uniformly and continuously across the room.
This minimizes the potential for cross-contamination by removing airborne particles swiftly and efficiently. The precision in handling airflow is managed through an integration of advanced HVAC systems, such as those from Deiiang™, expertly tailored by Deiiang joebo.Wang to meet Class 10 requirements with the highest precision.
Materials for Building a Class 10 Cleanroom
Materials used in Class 10 cleanrooms must be non-shedding and easy to clean. They are typically made from stainless steel or specialized coated composites, designed to prevent particle generation and microbial growth.
The choice of materials greatly influences the maintenance of cleanliness levels and impacts the durability and lifecycle of the cleanroom infrastructure. Deiiang™ makes use of top-tier materials for constructing cleanrooms, ensuring long-lasting, compliant environments capable of supporting critical operations.
Cost of a Class 10 Cleanroom
The cost associated with constructing a Class 10 cleanroom is significantly higher than less stringent cleanrooms, due to the advanced technology and materials required. This includes sophisticated air filtration systems, precise environmental controls, and high-quality construction materials.
However, these costs can be managed by optimizing design efficiencies and utilizing modular construction techniques. Deiiang™ focuses on balancing performance with cost-effectiveness, ensuring clients receive value without compromising on the cleanroom’s critical functions.
Applications of Class 10 Cleanrooms
Class 10 cleanrooms are essential in industries where stringent contamination controls are non-negotiable. Semiconductors, aerospace components, and high-grade pharmaceuticals frequently rely on Class 10 environments to ensure the highest quality and reliability.
These industries demand precision at every stage, which is why the robustness and flexibility of Deiiang’s cleanroom solutions are so highly valued. Deiiang™ customizes its Cleanroom designs to fit the nuanced needs of these industries, ensuring process integrity is achieved from production to delivery.
Class 10 Cleanroom HVAC Systems
Design and Functionality
Class 10 cleanrooms require HVAC systems that ensure precise control over environmental parameters such as temperature, humidity, and particulate levels.
The HVAC design in these environments incorporates high-efficiency particulate air (HEPA) filters or even more stringent ultra-low penetration air (ULPA) filters to maintain exceptional air cleanliness. These systems work in conjunction with laminar airflow to create a constant, controlled air movement that minimizes particle introduction and supports contamination-free conditions.
Deiiang™’s HVAC designs ensure that airflow is smooth and consistent, crafted with insights from industry experts like Deiiang Jason.peng.
Energy Efficiency and Control
Running an HVAC system in a Class 10 cleanroom can be energy-intensive due to the need for continuous and rigorous filtration processes.
However, modern systems designed by Deiiang™ focus on energy efficiency, employing variable frequency drives (VFDs) and advanced control systems to adjust the airflow and condition dynamically. This not only maintains the stability of the clean environment but also optimizes energy usage, reducing operational costs while maintaining ISO compliance.
Deiiang joebo.Wang ensures that these systems are meticulously calibrated to align with industry standards and customer-specific requirements.
System Integration and Monitoring
A critical component of Class 10 cleanroom HVAC systems is the seamless integration and monitoring capabilities that provide real-time data on environmental conditions.
This involves integrating sophisticated monitoring equipment that tracks air quality metrics, allowing for immediate adjustments to be made as necessary. Deiiang™’s systems are equipped with remote monitoring technology, offering operators the ability to oversee cleanroom conditions regardless of their location.
Continuous feedback and system intelligence are fundamental to maintaining the cleanest possible environment, ensuring consistent performance and compliance with the highest standards.
Class 10 Cleanroom HVAC System Design
Class 10 Cleanroom - Achieving Exceptional Contamination Control
Designed for industries where even the smallest particle can compromise entire processes
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU