Purpose
Establish a standard operating procedure for sedimentation bacteria detection in clean areas (rooms) of clean workshops to ensure that microbial detection in clean areas is carried out in accordance with specifications
Scope
Determination of sedimentation bacteria and verification of the environment in all clean areas (rooms) of the company.
Responsible person
The Quality and Technology Department is responsible for the implementation of this SOP.
Procedure
This test method adopts the sedimentation method, that is, the biological particles in the air are collected on the culture medium plate by the principle of natural sedimentation. After a period of time, under suitable conditions, they are allowed to multiply to visible colonies for counting. The number of colonies in the flat culture plate is used to determine the number of live microorganisms in the clean environment, and the cleanliness of the clean area (room) is evaluated on this basis. 4.2 Instruments and equipment used.
High-pressure steam sterilizer When using, it should be operated strictly in accordance with the "High-pressure steam sterilizer operating procedures" and instructions
Constant temperature incubator The thermometer of the incubator must be calibrated regularly
Petri dishes Use 90mmx15mm borosilicate glass petri dishes
Culture medium
Ordinary broth agar medium or other culture medium approved by pharmacopoeia
Preparation and sterilization of culture medium:
a.Place the Φ90mm petri dish in 121℃ wet heat sterilization for 20min or 180℃ dry heat sterilization for 2h.
b.Heat the culture medium to melt, and when it cools to 45℃, inject the culture medium into the petri dish under the requirements of aseptic operation, about 15ml per dish.
c.After the agar solidifies, invert the culture medium plate in a 30℃-35℃ constant temperature incubator for incubation
48h, if there is no colony growth on the culture medium plate, it can be used for sampling. The prepared culture medium plate should be stored in an environment of 2℃-8℃.
Test steps
1.Sampling method
Place the prepared culture dish according to the requirements of 5.4.1.2, open the cover of the culture dish, expose the surface of the culture medium for 0.5h, then cover the cover of the culture dish and turn it upside down. 4.3.2 Culture
2.After all sampling is completed, place the culture dish upside down in a constant temperature incubator for culture.Culture in a 36℃+1 incubator for no less than 48h.EACH batch of culture medium should have a control test to check whether the culture medium itself is contaminated. Three culture dishes can be selected for control culture in each batch.
3.Colony count
Count directly with the naked eye, mark or count on the colony counter, and then check with a 5-10x magnifying glass to see if there are any omissions.
If there are 2 or more colonies overlapping on the culture dish, they should still be counted as 2 or more colonies if they can be distinguished.
Precautions
1.Test equipment should be sterilized to ensure the reliability and correctness of the test
2.Take all measures to prevent human contamination of samples.
3.Keep detailed records of culture medium, culture conditions and other parameters.
4.Due to the wide variety of bacteria and their great differences, when counting, it is generally necessary to carefully observe the back or front of the culture dish with transmitted light, and do not miss the colonies growing on the edge of the culture dish. It is also necessary to pay attention to the difference between bacterial colonies and culture medium sediments, and use a microscope for identification when necessary.
5.Before sampling, the quality of each culture dish should be carefully checked. If it is found to be deteriorated, damaged or contaminated, it should be discarded.
Test rules
1.Test status
1.Before the sedimentation bacteria test, the temperature and humidity of the clean area (room) to be tested must meet the specified requirements, and the static pressure difference, ventilation frequency, and air flow rate must be controlled within the specified values.
2.Before the sedimentation bacteria test, the clean area (room) to be tested has been disinfected.
3.There are two test states: static and dynamic. The choice of test state must meet the production requirements and the test state must be indicated in the report.
2.Testers
1.Testers must wear work clothes that meet the environmental cleanliness level.
2.During static testing, there must be no more than two testers in the room.
3.Test time
1.For unidirectional flow, such as class 100 clean rooms and laminar flow workbenches, the test should start at least 10 minutes after the purification air conditioning system is operating normally.
2.For non-unidirectional flow, such as Class
10000 and class 100000 clean rooms, the test should start at least 30 minutes
after the purification air conditioning system is operating normally.
| area m² | Cleanliness level | |
| 100 | 100,000 | |
| <10 | 2~3 | 2 |
| ~<20 | 4 | 2 |
| ~<40 | 8 | 2 |
| ≥40~<100 | 16 | 2 |
| ≥100~<200 | 40 | 3 |
| ≥200~<400 | 80 | 6 |
| ≥400~<1000 | 160 | 13 |
| ≥1000~<2000 | 400 | 32 |
| 2000 | 800 | 63 |
4.Settling bacteria count
Number and basic layout of sampling points
1.Minimum number of sampling points
2.Layout of sampling points
The location of sampling points in the work area is about 0.8m~1.5m from the ground (slightly higher than the work surface).
Sampling points can be added at key equipment or key work activity range.
Detailed rules for sampling point locations are shown in the attached figure.
| Cleanliness level | The number of Φ90mm blood required for culture (based on sedimentation for 0.5h) |
| 100 | 14 |
| 10000 | 2 |
| 100,000 | 2 |
5.Records
The room temperature, relative humidity, pressure difference and test status should be recorded in the test report: the preparation of the test report is shown in the attached table.
6.Results
1.The number of colonies in each culture dish is obtained by counting method.
2.The calculation of the average number of colonies is shown in the following formula.
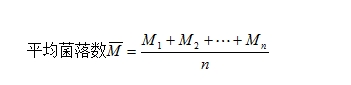
Where: M is the average colony count
M1 is the colony count of culture dish No. 1
M2 is the colony count of culture dish No. 2
Mn is the colony count of culture dish No. n
n is the total number of culture dishes.
7.Result evaluation
Use the average colony count to judge the microorganisms in the air of the clean area (room). The sedimentation bacteria (individuals/m2) of the 100,000-level clean area should be less than or equal to 10; the sedimentation bacteria (individuals/m2) of the 10000-level clean area should be less than or equal to 3; the sedimentation bacteria (individuals/m2) of the 100-level clean area should be less than or equal to 1.
The average colony count in the clean area (room) must be lower than the selected assessment standard.
If the average colony count in a clean area (area) exceeds the assessment standard, the area must be disinfected first, and then resampled twice, and the test results must be qualified.
| Monitoring Department | Monitoring date | Year Month Day | |||||||
| Test Status | Static port Dynamic Mouth | Report Date | Year Month Day | ||||||
| Cultivation temperature | Celsius | Cultivation time | hour | ||||||
| Colony count | 1 | 2 | 3 | 4 | Average | Evaluation results | |||
| Reviewer | |||||||||
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



