Foreword
This standard updates iso 14698-1 and ISO/TS 11133-1, replacing GB/T 16294-1996. Key changes include added sampling points, revised incubation conditions for culture dishes, and new daily monitoring guidelines for air microbial concentration in clean rooms.
Appendix A and Appendix B of this standard are normative appendices
Appendix C of this standard is an informative appendix.
Test method for sedimentation bacteria in clean rooms (areas) of pharmaceutical industry.

(Figure 1: Clean room)
1 Scope
This standard specifies the test conditions and test methods for sedimentation bacteria in clean rooms and clean areas of pharmaceutical industry.
This standard applies to the testing of sedimentation bacteria and environmental verification in clean rooms and clean areas, sterile rooms or local air purification areas (including clean workbenches) in the pharmaceutical industry.
2 Normative references
The clauses in the following documents become clauses of this standard through reference in this standard. For all dated references, all subsequent amendments (excluding errata) or revisions are not applicable to this standard. However, parties who reACH an agreement based on this standard are encouraged to study whether the latest versions of these documents can be used. For all undated references, the latest versions apply to this standard.
GBIT 16292-2010 Test method for floating particles in clean rooms (areas) in pharmaceutical industry
3 Terms and definitions
The following terms and definitions apply to this standard.
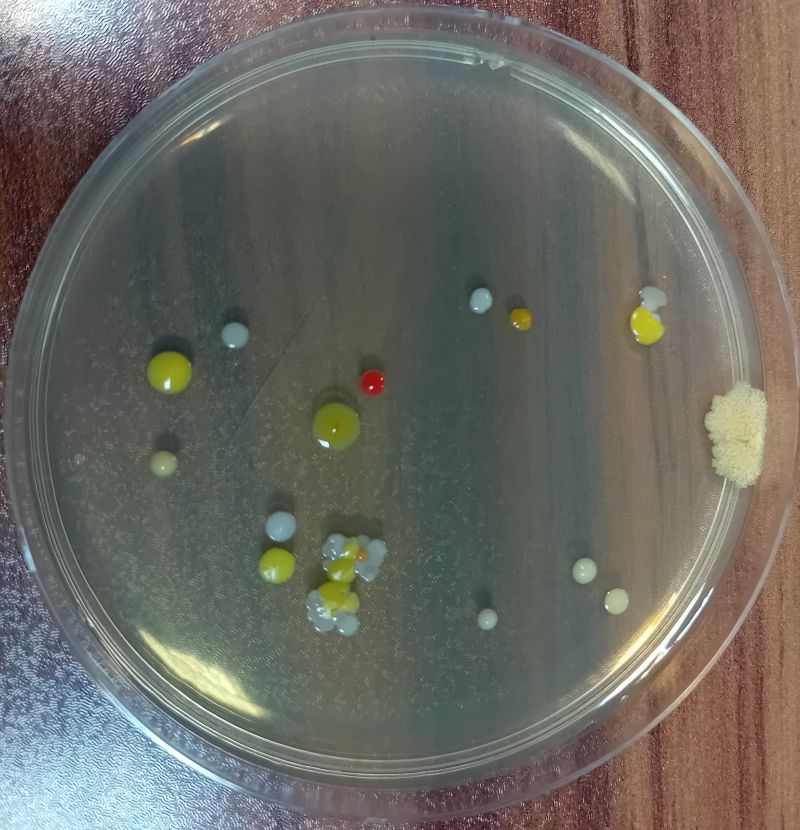
(Figure 2: Settling microorganisms)
3.1 Settling microbes Live microbial particles in the air are collected by the method mentioned in this standard, and propagated to visible colonies under suitable growth conditions through a special culture medium.
3.2 Settling microbe plate count
The number of settling microbes collected in the air by each plate culture dish within the specified time, expressed as / plate.
4 Test method
4.1 Method summary
This test method adopts the settling method, that is, the biological particles in the air are collected on the culture plate through the principle of natural sedimentation. After a period of time, under suitable conditions, they are allowed to multiply to visible colonies for counting. The number of colonies in the plate culture dish is used to determine the number of live microorganisms in the clean environment, and the cleanliness of the clean room (area) is evaluated based on this.
4.2 Personnel job and training
The test personnel of the clean room (area) should be trained in this profession and obtain the corresponding qualifications before they can perform the duties of the clean room (area) test, which includes the hygiene knowledge and basic microbiological knowledge involved.

(Figure 3: cleanroom staff training)
The testers in the clean room (area) should choose the clothing style that is suitable for the air cleanliness level requirements of the production operation. The outer clothes cannot be brought into the area above 100000 level.
4.3 Instruments
The instruments should include:
Petri dishes;
Culture medium (see Appendix B of this standard);
Constant temperature incubator:
High pressure steam sterilizer:
4.3.1 Petri dishes
Use petri dishes with specifications of pp90mmx15mm.
4.3.2 Culture medium
Soybean casein agar medium (TSA) or Sabouraud medium (SDA) or other user-approved and verified culture medium. The preparation method is shown in Appendix B
4.3.3 Constant temperature incubator
The constant temperature incubator must be calibrated regularly.
(Figure 4: High Pressure Steam Sterilizer)
4.4 Test steps
4.4.1 The surface of the culture medium must be strictly disinfected before testing.
4.4.2 Place the prepared culture dishes one by one according to the sampling point layout diagram, and then open the culture dish lids one by one from the inside to the outside to expose the surface of the culture medium to the air.
4.4.3 During static testing, the exposure time of the culture dish is more than 30 minutes; during Dynamic testing, the exposure time of the culture dish is no more than 4 hours.
4.4.4 After all sampling is completed, the culture dish is inverted and placed in a constant temperature incubator for incubation.
4.4.5 After sampling, the culture dish prepared with soybean casein agar medium (TSA) is incubated in a 30℃~35℃ incubator for no less than 2 days; the culture medium prepared with Sabouraud medium (SDA) is incubated in a 20℃~25℃ incubator for no less than 5 days after sampling.
4.4.6 Each batch of culture medium should have a control test to check whether the culture medium itself is contaminated. Three culture dishes can be selected for control culture in each batch.
4.5 Colony Count
4.5.1 Count, mark or count all colonies on the culture dish directly with the naked eye, and then use a 5-10x magnifying glass to check if there are any omissions.
4.5.2 If there are 2 or more colonies overlapping on the plate, they should still be counted as 2 or more colonies if they can be distinguished.
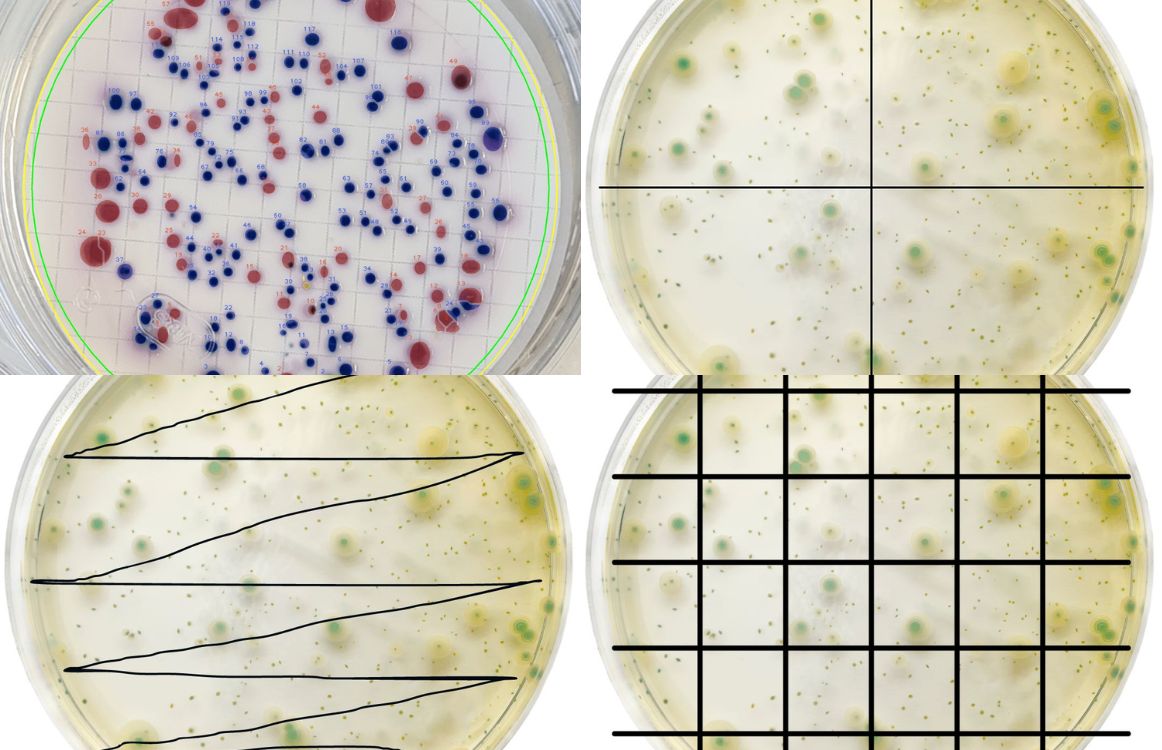
(Figure 5: Colony counting)
4.6 Things to Note
4.6.1 Test tools should be sterilized to ensure the reliability and correctness of the test.
4.6.2 Take all measures to prevent human contamination of samples.
4.6.3 Keep detailed records of culture medium, culture conditions and other parameters.
4.6.4 Since there are many types of bacteria and they vary greatly, when counting, generally use transmitted light to carefully observe the back or front of the culture dish. Do not miss the colonies growing on the edge of the culture dish, and pay attention to the difference between bacterial colonies and culture medium sediments. Use a microscope to monitor if necessary.
4.6.5 Before sampling, the quality of each culture dish should be carefully checked. If it is found to be deteriorated, damaged or contaminated, it should be discarded.
5 Test rules
5.1 Test conditions
Before testing, the relevant parameters of the clean room (area) should be pre-tested. Such tests will provide environmental conditions for testing sedimentation bacteria, for example: This pre-test may include:
Temperature and relative humidity testing. The temperature and relative humidity of the clean room (area) should be consistent with its production and process requirements (if there are no special requirements, the temperature should be 18℃~26° and the relative humidity should be 45%~65%). At the same time, it should meet the use range of the test instrument:
Indoor air supply volume or wind speed test, or pressure difference test:
Leakage test of high-efficiency filter
(Figure 6: clean room testing)
5.2 Test status
Both static and dynamic states can be tested.
During static testing, there should be no more than 2 testers in the room.
Before the sedimentation bacteria test, the user shall decide whether the clean room (area) under test needs to be disinfected in advance.
The test report should indicate the state used during the test and the number of testers in the room.
5.3 Test time
5.3.1 In the empty or static a test, for unidirectional clean rooms (areas), the test should start at least 10 minutes after the clean air conditioning system has been operating normally. For non-unidirectional clean rooms (areas), the test should start at least 30 minutes after the clean air conditioning system has been operating normally. In the static b test, for unidirectional clean rooms (areas), the test should start after the production operators have evacuated the site and after 10 minutes of self-cleaning; for non-unidirectional clean rooms (areas), the test should start after the production operators have evacuated the site and after 20 minutes of self-cleaning.
5.3.2 In the dynamic test, the time of production start and the test time must be recorded.
5.4 Calculation of the number of sedimentation bacteria colonies
5.4.1 Number of sampling points and their arrangement
5.4.1.1 Minimum number of sampling points
The minimum number of sampling points for sedimentation bacteria testing can refer to GB/T16292-2010.
5.4.1.2 Location of sampling points
The location of sampling points for sedimentation bacteria can refer to GB/T 16292-2010
The sampling point in the work area is about 0.8m~1.5m above the ground (slightly higher than the work surface)
Measuring points can be added at key equipment or key work activity range. The rules for the arrangement of sampling points are shown in Appendix A.
5.4.2 Minimum number of culture dishes
While meeting the minimum number of sampling points, the minimum number of culture dishes should also be met, see Table 1.
Table 1 Minimum number of culture dishes
| Cleanliness level | Minimum number of cultured blood (p90mm) |
| 100 | 14 |
| 10000 | 2 |
| 100000 | 2 |
| 300000 | 2 |
5.4.3 Number of sampling times
Each sampling point is generally sampled once.
5.4.4 Sampling precautions
5.4.4.1 For unidirectional clean rooms (areas) or air outlets, the sampling port of the sampler should face the direction of the airflow; for non-unidirectional clean rooms (areas), the sampling port should face upward.
5.4.4.2 When arranging sampling points, at least the return air outlet where dust particles are concentrated should be opened as much as possible.
5.4.4.3 When sampling, the tester should stand on the downwind side of the sampling port and move as little as possible:
5.4.4.4 All measures should be taken to prevent contamination during the sampling process and other possible contamination of the sample.
5.4.4.5 When the culture dish is used for testing, in order to avoid the impact caused by the transportation or moving process of the culture dish, it is advisable to carry out a control test at the same time. Take one control dish each time or in each area, operate it in the same way as the sampling dish but do not need to be exposed to the sample, and then put it into the incubator together with the culture dish (TSA or SDA) after sampling. The result should be no colony growth.
5.5 Records
The test report should include the following:
Name and address of the tester, test date:
Test basis:
Plane location of the clean room (area) being tested (mark the plane location of adjacent areas if necessary):
Description of the test instrument and its test method: including test environment conditions, number of sampling points and layout diagram, number of tests, or possible changes in test methods, test instrument calibration certificate, etc.; if it is a dynamic test, the number and location of on-site operators, number and location of on-site equipment, etc. should also be recorded:
Test results: including all statistical calculation data.
5.6 Result calculation
5.6.1 Use the counting method to obtain the number of colonies in each culture dish.
5.6.2 Calculation of the average number of colonies of sedimentation bacteria at each measuring point, see formula (1).
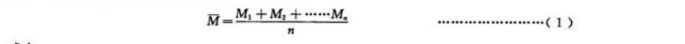
5.7 Result evaluation
5.7.1 The average number of colonies of sedimentation bacteria at each measuring point must be lower than the limit in the selected evaluation standard.
5.7.2 During the static test, if the average colony count of sedimentation bacteria at a certain measuring point exceeds the assessment standard, the sample should be resampled twice, and the results of both tests must be qualified to be judged as compliant.
5.8 Daily monitoring
For the monitoring of sedimentation bacteria, it is advisable to set correction limits and warning limits to ensure that the microbial concentration in the clean room (area) is controlled. Regular testing should be carried out to check the microbial load and the effectiveness of the disinfectant, and to make a trend analysis.
This method can be used for both static and dynamic monitoring. For the sampling frequency of sedimentation bacteria, if the following situations occur, modification should be considered. After evaluating the following situations, the detection frequency of other items should also be determined:
Continuously exceeding the correction limit and warning limit;
The shutdown time is longer than expected:
Contamination is found in the key area;
Any major maintenance of the air purification system during production;

(Figure 7: Clean room disinfection)
Daily operation records reflect trending data;
Changes in disinfection procedures;
Accidents causing biological contamination, etc.;
When production equipment has major maintenance or equipment is added:
When there are major changes in the structure or regional distribution of the clean room (area).
Appendix A (Normative Appendix) Sampling point arrangement in clean room (area)
A.1 The sampling point arrangement in clean room (area) should be uniform to avoid too sparse sampling points in local areas. The following diagram of sampling point arrangement for multi-point sampling can be used as a reference (see Figure A.1)
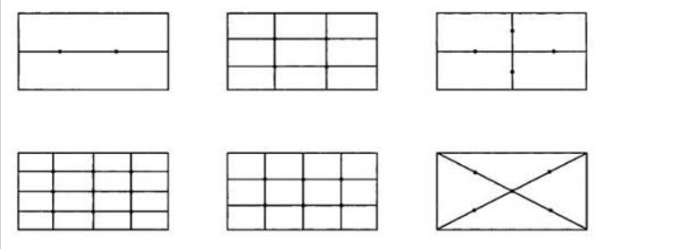
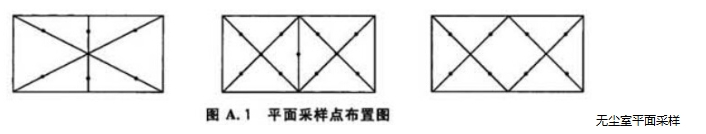
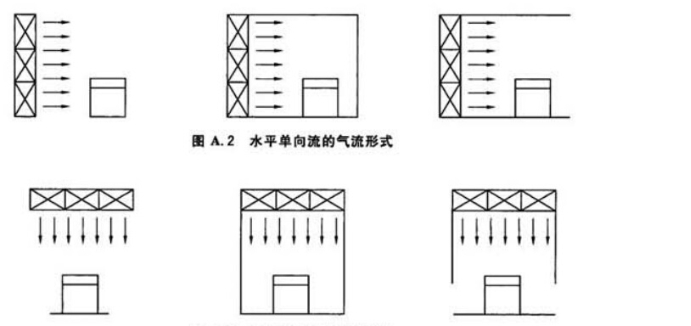
A.2 In the 100-level unidirectional flow area, the sampling points of the clean workbench or local air purification facilities should be arranged on the working surface facing the airflow direction. The airflow form can refer to Figure A.2 and Figure A.3.
Appendix B (Normative Appendix) Sterilization and preparation of culture medium
B.1 Sterilization and preparation of soybean casein agar medium (TSA)
B.1.1 The culture medium uses soybean casein agar medium, which can be prepared according to the following prescription, or a dehydrated culture medium produced according to the prescription that meets the requirements can be used. After preparation, sterilize according to the validated sterilization procedure specified in the culture medium.
B.1.2 Formula of soybean casein agar culture medium
Casein pancreatic digest
Soybean powder papain digest
Sodium amide
Agar
Purified water
B.2 Sterilization and preparation of Sabouraud agar (SDA) culture medium
Glucose.
Casein pancreatic digest
Peptic digest of animal tissue
Mix in equal amounts
Take the above ingredients except agar, mix, dissolve with slight heat, adjust the pH value to 5.6±0.2 after sterilization, add agar, and pour about 20mL into a sterile plate (o90mml)) under the requirements of bacterial operation. Cover and place at room temperature until solidified.
B.3 Culture and storage of culture medium plates The prepared culture medium plates should be stored at 2℃~8℃, generally for one week or according to the standards provided by the manufacturer. Use appropriate methods to mark the name of the culture medium and the date of preparation on the plate.
Note 1): If other specifications of plates are used, the amount of culture medium can be appropriately increased or decreased to form an agar layer at least 2 mm thick in the plate.
Appendix C (Informative Appendix) Technical requirements for sedimentation bacteria in clean rooms (areas)
Technical requirements for sedimentation bacteria in clean rooms (areas)
| Cleanliness level | Australian TGA CGMP | EU CGMP Appendix | US FDA CGMP | Drug production quality management standards | ||
| φ90mm CFU/4ha | φ90mm CFU/4ha | φ90mm CFU/4ha | Sedimentation bacteria/dish, 0.5h | |||
| 100 | Alevel | <1 | Alevel | <1 | <1 | ≤1 |
| 1000 | Blevel | ≤5 | Blevel | ≤5 | ≤3(1000level) | _ |
| 10000 | Clevel | ≤50 | Clevel | ≤50 | ≤5 | ≤3 |
| 100000 | Dlevel | ≤100 | Dlevel | ≤100 | ≤50 | ≤10 |
| a refers to the exposure time of the culture dish not exceeding 4h, expressed as the average number of colonies. | ||||||
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



