Good Manufacturing Practice (GMP) is a cornerstone of pharmaceutical production, ensuring that products are consistently manufactured and controlled to the quality standards appropriate for their intended use.
Understanding GMP (Good Manufacturing Practice)

What is GMP?
GMP serves as a robust quality assurance system designed to minimize production risks such as contamination and errors, thereby safeguarding product safety, purity, and efficacy. It is a systematic approACH to ensuring that pharmaceutical products meet stringent quality standards at every stage of the manufacturing process.
Scope of GMP Regulations
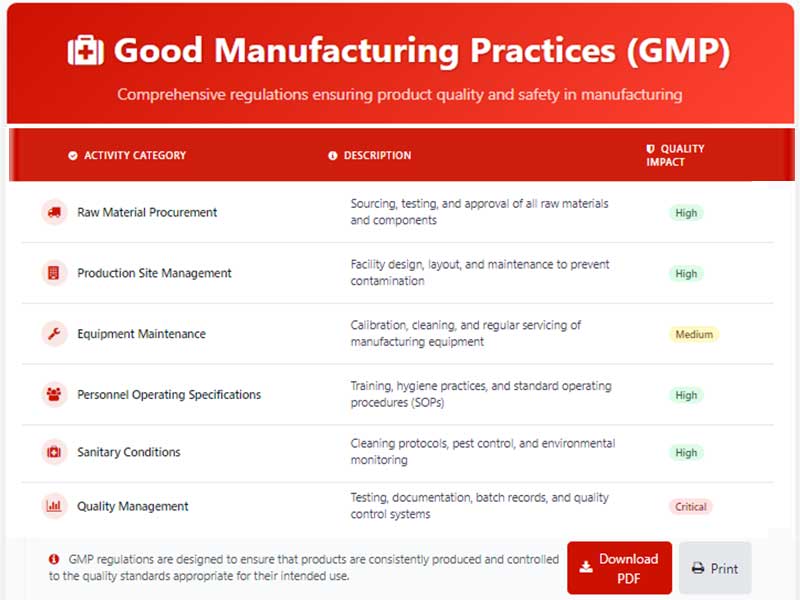
GMP regulations cover a broad spectrum of activities, including:
- Raw material procurement
- Production site management
- Equipment maintenance
- Personnel operating specifications
- Sanitary conditions
- Quality management
This comprehensive approach ensures that every aspect of the manufacturing process is controlled and monitored to maintain product quality and safety.
Why is GMP Important?
GMP is vital for several reasons. First and foremost, it protects patient safety and public health by ensuring that pharmaceutical products are safe, effective, and of high quality. Second, compliance with GMP regulations helps manufacturers avoid penalties and maintain their market access. Finally, GMP ensures product integrity and efficacy, which is crucial for building trust with healthcare professionals and patients.
GMP Standards and Requirements
Here are the key areas of GMP, presented for clarity:
| GMP Aspect | Focus |
| People | Training, hygiene, roles |
| Products | Material control, specifications |
| Procedures | SOPs, change control |
| Processes | Validation, maintenance, sanitation |
| Site | cleanrooms, monitoring, waste |
GMP: People - Human Factors in the Production Process
The human element is critical in GMP. Personnel must be adequately trained and qualified to perform their assigned tasks. Hygiene and personal behavior are also essential to prevent contamination. Clear roles and responsibilities are necessary for effective GMP compliance.
GMP: Product - Ensure Quality from Beginning to End
Ensuring product quality starts with the selection and control of raw materials. Product specifications and testing are crucial to verify that the final product meets the required standards. Traceability and documentation are also essential to track the product's journey throughout the manufacturing process.
GMP: Procedures - Standardized Operations
Standardized operations are achieved through the development and implementation of Standard Operating Procedures (SOPs). Change control and deviation management are necessary to handle any unexpected events or changes to the manufacturing process. Documentation practices ensure that all activities are recorded accurately and completely.
GMP: Process - Integrated Controls in Production
Process validation and monitoring are essential to ensure that the manufacturing process consistently produces a product of the required quality. Equipment qualification and maintenance are necessary to keep equipment in good working order. Cleaning and sanitation procedures prevent contamination.
GMP: Site - Facility Design and Maintenance
The design and maintenance of the manufacturing facility are critical for GMP compliance. Cleanroom requirements, including design, materials, airflow, and particle control, must be met. Environmental monitoring ensures that the facility remains within the required parameters. Waste management prevents contamination and maintains a safe working environment.
Application of GMP in Various Industries
GMP in Pharmaceutical Production
In pharmaceutical production, GMP guidelines are particularly stringent. Aseptic processing and sterilization are crucial for sterile products. Quality control and testing ensure that the final product meets the required specifications. Packaging and labeling requirements protect the product from contamination and provide accurate information to the user.
GMP in the Food Industry
Good Manufacturing Practices (GMP) are essential in the food industry for ensuring product safety and quality. Implementing GMP reduces contamination risks and enhances consumer trust by up to 60%. It involves proper hygiene, equipment use, and staff training. Regular audits and documentation improve traceability and operational efficiency, reducing waste by 15% and increasing consistency by 25%, as supported by case studies. By meeting regulatory standards like the FDA and EFSA, GMP aids in sustainable and profitable operations.
GMP in Cosmetics and Medical Devices
In the cosmetics industry, Good Manufacturing Practices (GMP) ensure product safety, reducing contamination and recalls by up to 40%. GMP involves hygiene protocols, equipment maintenance, and staff training. In medical devices, GMP ensures safety and effectiveness by reducing failure rates by 30% through rigorous documentation and quality control. Adhering to GMP meets regulatory standards like the FDA and enhances consumer trust and global competitiveness.
GMP in cleanroom environments
Contamination Control in Cleanrooms
Contamination control in cleanrooms is achieved through several measures. Air filtration using HEPA/ULPA filters removes airborne particles. Cleaning and disinfection procedures eliminate surface contamination. Personnel dress and behavior standards prevent the introduction of contaminants.
cleanroom design and Construction: GMP Considerations
cleanroom design and construction must meet GMP requirements. Materials and surface treatments must be easy to clean and disinfect. Airflow patterns and pressure differentials control the movement of air and prevent contamination. Partitioning and isolation separate different areas of the cleanroom to prevent cross-contamination.
Cleanroom Operating Standards
Cleanroom operating standards include particle monitoring and control, temperature and humidity control, and personnel access procedures. These standards ensure that the cleanroom environment remains within the required parameters.
Cleanroom Performance Verification and Monitoring
Cleanroom performance is verified and monitored through airflow visualization studies, particle counting and microbial monitoring, and cleaning and disinfection verification. These activities ensure that the cleanroom is performing as intended and that any deviations are quickly identified and corrected.
Cleanroom Workflow and Efficiency
Efficient workflow is essential in cleanrooms. Material flow and isolation prevent cross-contamination. Equipment placement and access minimize the risk of contamination. Waste removal prevents the accumulation of contaminants.
Cleanroom Regulatory Compliance
Cleanrooms must comply with several regulations, including iso 14644 standards, EU GMP Annex 1 (specifically related to aseptic production), and FDA guidance.
GMP System Components
GMP "Hardware": Physical Conditions and Infrastructure
The application of Good Manufacturing Practice (GMP) in pharmaceutical manufacturing is essential to ensure the quality, safety and efficacy of medicines.GMP encompasses a comprehensive quality management system that governs the entire lifecycle of pharmaceutical production, from raw material procurement to distribution. It requires strict adherence to validated processes, proper documentation and stringent quality control measures.
GMP "Software": The Intangible Elements of Quality
The "software" of a GMP system includes the intangible elements of quality, such as personnel training and competence, the Quality Management System (QMS), and Standard Operating Procedures (SOPs).
The Future of GMP in Pharmaceutical and Other Industries
GMP will continue to evolve in the future, driven by advances in technology and changes in regulatory requirements. As the pharmaceutical and other industries become more complex, GMP will play an increasingly important role in ensuring product quality and safety. By maintaining high standards, manufacturers can contribute to public health and build trust with patients and healthcare professionals.
gmp documentation - Ensuring Quality and Safety in Drug Production
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


