What is the Difference Between ISO 7 and 8?

1. Cleanliness Levels
The primary distinction between ISO 7 and iso 8 lies in their cleanliness levels. ISO 7 environments allow a maximum of 352,000 particles per cubic meter of air that are 0.5 micrometers and larger. In contrast, ISO 8 environments permit up to 3.5 million particles per cubic meter for the same particle size. This significant difference in allowable particulate concentration indicates that ISO 7 environments are more stringent and suitable for processes requiring higher levels of cleanliness.
2. Environmental Controls
iso 7 cleanrooms require more advanced environmental control systems. They must maintain a temperature range of 20-24°C and a relative humidity of 30-60%. Moreover, air change rates are crucial; ISO 7 typically requires a minimum of 30 air changes per hour.

3. Application Areas
ISO 7 cleanrooms are commonly used in applications where contamination control is critical, such as in the manufacture of sterile pharmaceuticals, biotechnology products, and advanced microelectronics. In contrast, ISO 8 environments are often used in less sensitive applications, such as assembling medical devices, packaging pharmaceuticals, and in some food processing situations. The choice of iso class directly impacts product quality and safety.
4. Compliance Requirements
Compliance with ISO 7 and iso 8 standards necessitates regular monitoring and validation of the cleanroom environment. ISO 7 requires more rigorous testing and documentation compared to ISO 8. This includes particle counting, microbial testing, and monitoring of airflow patterns. As a result, facilities operating at ISO 7 must invest more in equipment and staff training to maintain compliance.
| Feature | ISO 7 | ISO 8 |
| Maximum Particle Count | 352,000 particles | 3.5 million particles |
| Air Change Rate | 30+ changes/hour | 20 changes/hour |
| Temperature Range | 20-24 | 20-24 |
| Relative Humidity | 30-60% | 30-60% |
| Typical Applications | Sterile pharmaceuticals | Medical device assembly |
| Compliance Requirements | More stringent | Less stringent |
Is ISO 7 a Class C?
ISO 7, classified as a Class C cleanroom under EU GMP guidelines, maintains controlled cleanliness for non-sterile pharmaceutical processes. It suits activities like preparing non-sterile APIs and packaging, ensuring product quality and safety while meeting regulatory standards.
What are the Rules for ISO 7?

1. Air Cleanliness: ISO 7 cleanrooms must maintain a maximum of 352,000 particles per cubic meter for 0.5 micrometer particles.
2. Temperature and Humidity Control: The temperature should be kept between 20-24°C, with humidity levels maintained between 30-60%.
3. Air Change Rate: A minimum of 30 air changes per hour is required to ensure effective air circulation and reduce particle concentration.
4. Personnel Training: Staff must be trained in cleanroom protocols, including gowning procedures, to minimize contamination risks.
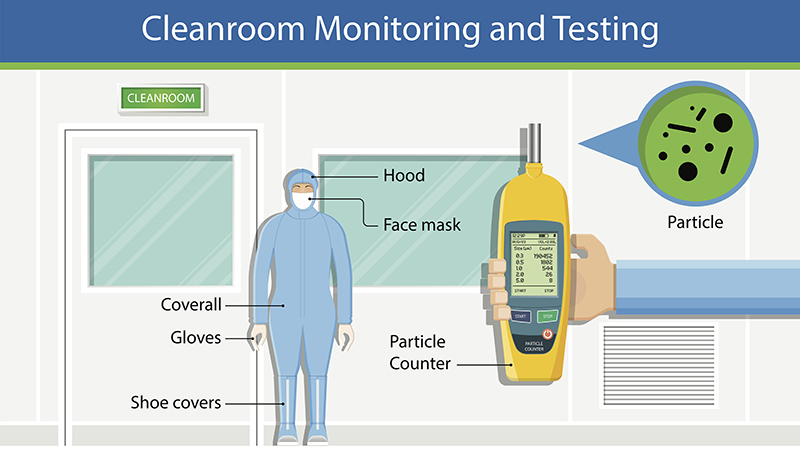
5. Monitoring and Validation: Regular monitoring of particulate levels, airflow patterns, and microbial contamination is essential. Validation of cleanroom performance should occur periodically.
6. Equipment Maintenance: All equipment used in ISO 7 environments must be regularly cleaned and maintained to prevent contamination.
7. Gowning Procedures: Strict gowning protocols must be followed, including the use of cleanroom garments, gloves, masks, and shoe covers.
8. Entry and Exit Protocols: Controlled access to the cleanroom is crucial, with procedures in place for entering and exiting to minimize contamination risks.
What is ISO 8 Used For?
iso 8 cleanrooms are designed for environments where the risk of contamination is lower than in ISO 7 settings but still requires control over airborne particulates. These cleanrooms are suitable for a variety of applications, particularly in industries that demand a controlled environment but do not require the stringent conditions of ISO 7.

Pharmaceutical Packaging
One of the primary uses for iso 8 cleanrooms is in the packaging of pharmaceutical products. While these products may not require sterile conditions, maintaining a lower level of particulate contamination is essential to ensure product integrity and safety. ISO 8 cleanrooms help in protecting products from dust, lint, and other airborne particles during the packaging process.
Medical Device Manufacturing
ISO 8 cleanrooms are also commonly used in the medical device industry. Here, the assembly and testing of devices that may come in contact with patients occur. The controlled environment helps prevent contamination that could interfere with the functionality or safety of the devices. While sterility may not be a primary concern, reducing particulate contamination is vital to comply with regulatory standards.
Food Processing and Other Industries
Beyond pharmaceuticals and medical devices, ISO 8 cleanrooms find applications in food processing and other industries where cleanliness is important but not as critical as in ISO 7 settings. In these environments, the focus is on preventing contamination that could affect product quality and safety, making ISO 8 an appropriate choice.
How to Use ISO 8?
Using ISO 8 cleanrooms effectively involves adhering to established protocols and ensuring that cleanliness is maintained. Here are the key steps to ensure proper usage:

1. Pre-Entry Protocol
Before entering the ISO 8 cleanroom, personnel should undergo a thorough gowning process. This includes wearing appropriate cleanroom garments, gloves, hair covers, and shoe covers to minimize contamination risks.
2. Air Quality Monitoring
Continuous monitoring of airborne particles is essential. Utilize particle counters to ensure that the cleanroom maintains the required iso 8 standards for air cleanliness.
3. Equipment Maintenance
Regular cleaning and maintenance of all equipment used within the cleanroom are crucial. Ensure that surfaces, tools, and machinery are sanitized to prevent contamination.
4. Workflow Management
Design workflows to minimize movement and potential contamination. Limit the number of personnel in the cleanroom at any given time to reduce the risk of airborne particles being introduced.

5. Regular Training
Conduct regular training sessions for all personnel working in the ISO 8 environment. This training should cover cleanroom protocols, equipment handling, and contamination prevention techniques.
6. Validation and Compliance
Implement a schedule for regular validation checks to ensure that the cleanroom continues to meet ISO 8 standards. This includes testing for particulate levels, assessing airflow patterns, and verifying cleanliness.
7. Documentation
Maintain thorough documentation of all monitoring, maintenance, and validation activities. This documentation is essential for compliance with regulatory standards and for ensuring continuous improvement in cleanroom practices.
What are the ISO 7 and 8 Gowning Requirements?
Introduction to ISO Classifications
ISO classifications are critical in maintaining cleanliness and preventing contamination in controlled environments, such as cleanrooms in pharmaceutical and biotechnology industries. ISO 7 and ISO 8 are two levels of cleanroom classifications defined by ISO 14644-1, characterizing the maximum allowable levels of airborne particulate contamination.

Gowning Requirements for ISO 7
a. Overall Gowning Protocol: Personnel entering an ISO 7 cleanroom must follow strict gowning protocols to minimize contamination. This includes wearing a full-body gown that covers all personal clothing and skin.
b. Material Specifications: The gowns should be made from non-linting, low-particulate materials that do not shed fibers or particles. Common materials include polyester or specialized cleanroom fabrics.
c. Head and Hair Covers: A hair cover, such as a bouffant cap, must be worn to prevent hair shedding. Additional head coverings may be necessary to cover facial hair.
d. Face Masks: Masks are essential in ISO 7 environments to prevent respiratory droplets from contaminating the cleanroom atmosphere. Surgical masks or N95 respirators are typically required.
e. Gloves: Double gloving is recommended, with the outer gloves being made of nitrile or latex. Gloves must be inspected for tears or defects prior to use.
f. Footwear: Cleanroom shoes or shoe covers must be worn to prevent the transfer of contaminants from outside the cleanroom.

Gowning Requirements for ISO 8
a. Gowning Essentials: While the gowning requirements for ISO 8 are slightly less stringent than for ISO 7, they still require a cleanroom gown that covers personal attire adequately.
b. Material and Design: The gowns should also be made from low-particulate materials, but may allow for less stringent specifications compared to ISO 7.
c. Hair and Beard Covers: Hair covers are mandatory in ISO 8, and beard covers are required for personnel with facial hair to limit contamination.
d. Masks: While masks are recommended, the type and necessity can vary based on the specific processes and risks within the ISO 8 cleanroom.
e. Gloves: Single gloves may be acceptable in ISO 8, but they should still be made of cleanroom-compatible materials, ensuring they are free from defects.
f. Footwear Guidelines: Similar to ISO 7, cleanroom shoes or shoe covers are required to maintain cleanliness.
What PPE is Required for ISO 8?

In ISO 8 cleanrooms, PPE is essential to minimize contamination risks. Personnel must wear low-particulate gowns, hair and beard covers, face masks, gloves, and cleanroom footwear to maintain a safe and clean environment, ensuring compliance with industry standards.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


