GMP Cleanliness Classification
Ensuring Pharmaceutical Purity and Safety
GMP cleanliness classification is an essential framework guiding the pharmaceutical industry towards maintaining stringent cleanliness standards necessary for ensuring product safety and efficacy.Primarily outlined in EU GMP Annex 1, these classifications dictate the environmental controls needed for sterile medicinal production. Paired with ISO 14644-1, these guidelines ensure cleanrooms operate at optimal conditions, minimizing contamination risks.

Understanding GMP CleanRoom Classifications
GMP cleanroom classifications are divided into four grades: A, B, C, and D. Each grade serves a specific purpose in maintaining the required cleanliness for pharmaceutical production.
Grade A
- Highest cleanliness level
- Equivalent to ISO 5
- Critical operations like aseptic filling
- Particle concentration: ≤1 particle/m³ at ≥0.5μm
Grade B
- Background for Grade A areas
- Also aligns with ISO 5
- Primarily "at rest" condition
- Facilitates aseptic preparation
Grade C
- Similar to ISO 7
- Handles sterile product preparation
- Less stringent cleanliness requirements
- Suitable for preparation stages
Grade D
- Matches ISO 8
- Used for initial sterile product preparation
- Cleaner than typical environments
- Foundational cleanliness level
Key Aspects of GMP Cleanliness
One of the primary aspects of GMP cleanliness revolves around particle concentration limits. EU GMP Annex 1 delineates these specifications for both "at rest" and "in operation" states, providing clear parameters to meet cleanliness standards.
Particle Concentration Limits
| Grade | At Rest (≥0.5μm) | In Operation (≥0.5μm) |
|---|---|---|
| A | ≤1 particle/m³ | ≤3,500 particles/m³ |
| B | ≤3,500 particles/m³ | ≤350,000 particles/m³ |
| C | ≤350,000 particles/m³ | ≤3,500,000 particles/m³ |
| D | ≤3,500,000 particles/m³ | Not defined |

Particle concentration monitoring in a GMP cleanroom
Correlation with ISO Standards
GMP grades are approximately correlated with iso 14644-1 classes, ensuring a streamlined understanding of cleanliness levels worldwide. This integration helps facilities apply consistent cleanliness measures across various regions, facilitating international operations and compliance.

Visual representation of GMP and ISO cleanliness correlation
GMP-ISO Correlation Table
- GMP Grade A ↔ ISO Class 5
- GMP Grade B ↔ ISO Class 5 (at rest)
- GMP Grade C ↔ ISO Class 7
- GMP Grade D ↔ ISO Class 8
Maintenance and Systems
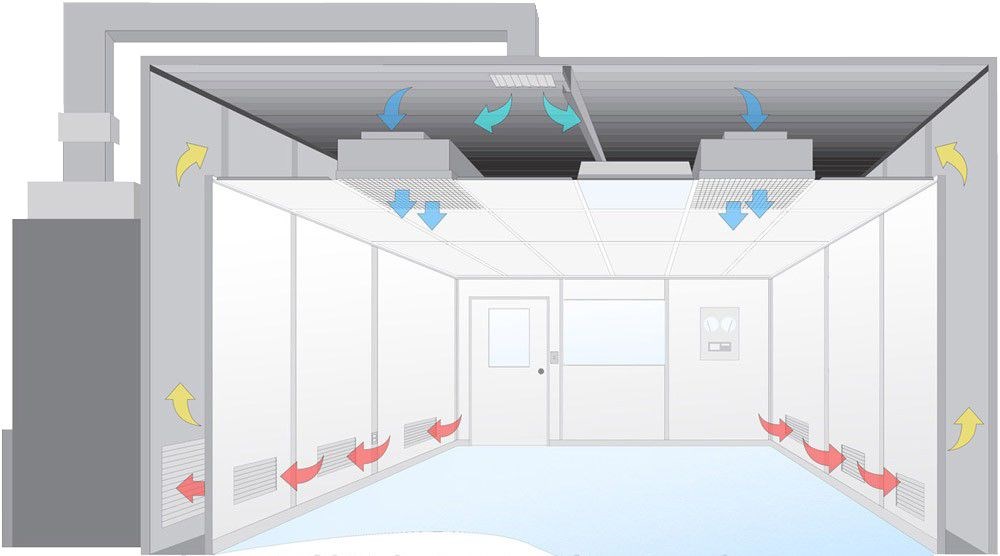
Maintaining these GMP grades involves sophisticated air circulation systems equipped with HEPA or ULPA filters that capture particulates to extremely low levels. Proper gowning and cleaning practices, complemented by sticky mats and airlocks, are integral to uphold these classified environments.
Key Maintenance Components
- HEPA/ULPA Filters: Capture 99.97%+ of particles ≥0.3μm
- Air Circulation Systems: Maintain consistent air flow and pressure
- Gowning Protocols: Specialized clothing to minimize particle shedding
- Cleaning Regimens: Scheduled cleaning with approved agents
- Sticky Mats: Trap particles from footwear
- Airlocks: Control air exchange between areas

HEPA filter installation in A CleanRoom air handling system
Material Requirements
GMP regulations specify the installation of smooth, waterproof, and easy-to-clean materials for constructing cleanrooms. These materials prevent contaminant accumulation and support effective cleaning protocols.

Stainless steel surfaces in a GMP compliant cleanroom
Preferred Cleanroom Materials
- •Stainless Steel: Corrosion-resistant, smooth surface
Epoxy flooring: Seamless, waterproof, chemical-resistant- PVC Wall Panels: Smooth, non-porous surface
Polyurethane Coatings: For walls and ceilings
Conclusion
GMP cleanliness classification serves as the foundation for maintaining controlled environments in the pharmaceutical industry. From Grade A's critical applications to the foundational requirements of Grade D, understanding these categories is vital for ensuring that cleanrooms operate efficiently and safely. Compliance with these standards, alongside ISO correlations, enables facilities to maintain high-quality production standards.
Common Questions About GMP Cleanliness Classification
1. What differentiates Grade A from Grade b Cleanrooms?
Grade A focuses on critical operations with stricter controls, while Grade B supports as a background environment with moderate controls. Grade A is for areas where product is exposed, such as during aseptic filling, while Grade B surrounds these areas to maintain a controlled buffer zone.
2. How do GMP and ISO standards relate in cleanroom management?
GMP Standards align with ISO classes for consistent cleanliness measures globally. This correlation allows for a universal understanding of cleanliness levels, making it easier for international pharmaceutical companies to maintain compliance across different regions.
3. Why are HEPA/ULPA filters critical in GMP Cleanrooms?
HEPA (High-Efficiency Particulate Air) and ULPA (Ultra-Low Penetration Air) filters are critical because they capture particles effectively, ensuring controlled environments remain clean. HEPA filters remove at least 99.97% of particles 0.3 microns and larger, while ULPA filters are even more efficient, removing 99.999% of particles 0.12 microns and larger.
4. What role do airlocks play in maintaining GMP Standards?
Airlocks minimize contamination risk by controlling air exchange between classified areas and non-controlled environments. They act as buffer zones, preventing direct airflow that could introduce particles, and allow for personnel and material transfer while maintaining pressure differentials to ensure cleanliness integrity.
5. Why is material choice crucial in cleanroom construction?
Material choice is crucial because durable, easy-to-clean materials prevent contaminant build-up, maintaining high cleanliness levels. Smooth, non-porous surfaces resist particle adhesion and are easier to disinfect, ensuring that the cleanroom environment can be effectively maintained and validated for GMP compliance.
GMP Cleanliness Classification - Ensuring Pharmaceutical Quality and Safety
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU