Clean Room Grades: Understanding Standards and Classifications
Clean rooms are essential environments in industries that require stringent control over airborne contaminants, such as pharmaceuticals, electronics manufacturing, biotechnology, and aerospace. These controlled environments are classified based on the concentration of particles per cubic meter, which determines the cleanliness level. This article explores the clean room grading system, focusing on key grades, standards, and their applications.
1. Clean Room Classification System
Clean room grades are defined by the number and size of particles permitted per unit of air volume. The most commonly used classification system is ISO 14644-1, an international standard that defines the clean room levels by particle count for different particle sizes. For example, ISO1 allows for no particles larger than 0.1 microns, while Class 9 has a much higher permissible particle count.
iso 14644-1: Classifies clean rooms from Class 1 (most stringent) to Class 9 (least stringent).
Particle Sizes: Typically measured in 0.1μm, 0.5μm, and 5.0μm particles.
Particle Count Limits: Vary depending on class, from 1 particle/m³ in Class 1 to 3,520,000 particles/m³ in Class 9.
2. Particle Concentration Limits
Each clean room class is assigned a maximum particle concentration that defines its cleanliness. For instance, Class 10,000 has a maximum allowable concentration of 3,520,000 particles of 0.5 microns per cubic meter of air, while Class 100,000 allows up to 35,200,000 particles per cubic meter. These concentrations determine the type of processes or industries that can operate in each class of clean room.
Class 1: Max 1 particle/m³ of 0.1µm size.
Class 10,000: Max 3,520,000 particles/m³ of 0.5µm size.
Class 100,000: Max 35,200,000 particles/m³ of 0.5µm size.

3. Applications of Different Clean Room Grades
Clean room grades determine the type of operations and industries they support. Class 1 to Class 100 are commonly used in high-precision industries such as semiconductor manufacturing, pharmaceuticals, and aerospace. On the other hand, Class 100,000 is often suitable for less sensitive applications like food processing or general laboratory environments.
Class 1-10: Used in semiconductors, pharmaceuticals, and biotechnology.
Class 10,000-100,000: Used for medical device assembly, food packaging, and general research.
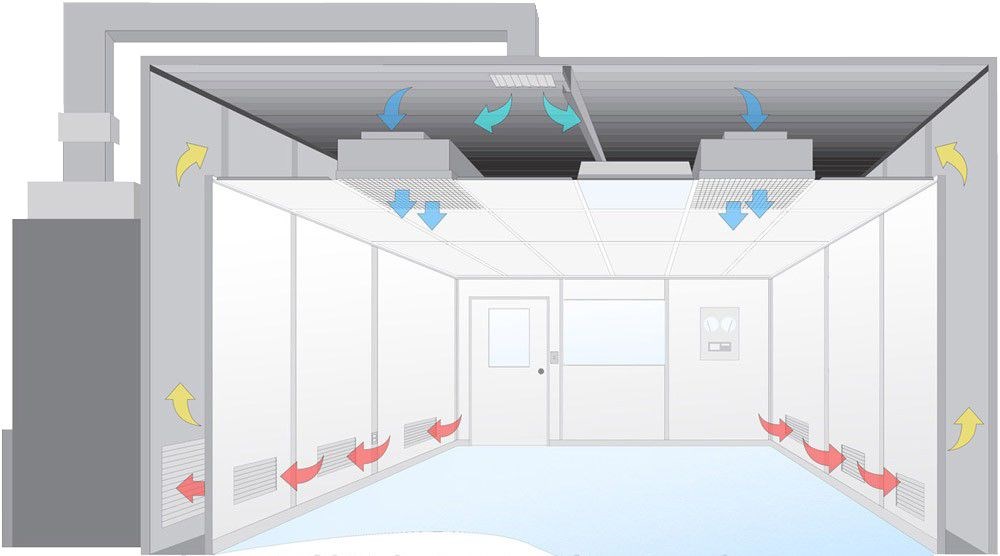
4. Airflow and Filtration Requirements
Airflow design is crucial for maintaining the cleanliness of a clean room. Laminar flow (unidirectional airflow) is typically used in high-grade clean rooms, such as Class 1 to Class 100. This ensures that air flows in a uniform direction, reducing the chances of particle contamination. Filtration systems, often HEPA (High-Efficiency Particulate Air) or ULPA (Ultra-Low Penetration Air) filters, are used to remove airborne contaminants.
Class 1 to Class 10: Laminar airflow and ULPA/HEPA filters are used.
Class 100 to Class 100,000: Typically HEPA filters and less stringent airflow patterns.

5. Maintenance and Monitoring Requirements
Clean rooms require regular monitoring and maintenance to ensure they remain within the specified cleanliness levels. For example, Class 1 clean rooms need continuous monitoring of particle counts, airflow, and humidity, while Class 100,000 may have less frequent checks. Routine maintenance includes changing filters, cleaning surfaces, and checking HVAC systems.
Class 1 to Class 10: Continuous monitoring and maintenance required.
Class 100,000: Periodic monitoring and maintenance.
6. Relevant Standards for Clean Room Design
Several international standards guide the design, operation, and maintenance of clean rooms. These standards ensure that the environments meet the necessary particle count requirements and are suitable for their specific applications. Key standards include:
ISO 14644-1: CleanRoom Classification and particle count standards.
iso 14644-2: Monitoring and control of clean room environments.
ISO 14644-4: Design and construction requirements for clean rooms.
iso 14644-5: Operation and maintenance of clean rooms.
How do you classify a clean room?

Clean Room Standards
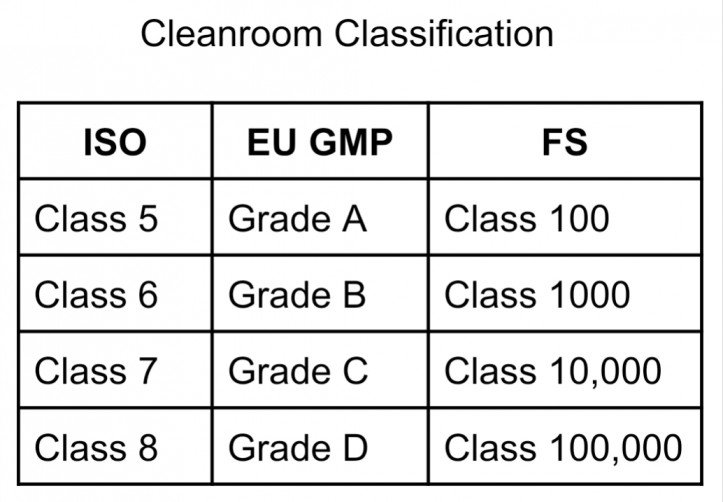
Clean rooms are classified based on the concentration of airborne particles. Standards like ISO and Federal Standard 209E provide guidelines for categorizing rooms by their cleanliness levels.
ISO Classification System
The ISO classification ranges from Class 1 to Class 9, with Class 1 being the cleanest. Each class defines limits for particle counts per cubic meter, establishing the cleanliness required for specific applications.
What is GMP grade?

Definition of GMP
GMP stands for Good Manufacturing Practices, which are guidelines ensuring consistent quality in production. They are essential for industries such as pharmaceuticals, food, and cosmetics.
GMP Grades Explained
GMP grades indicate the level of cleanliness and control required in manufacturing environments. Higher GMP grades necessitate more stringent protocols to minimize contamination risks.
Importance of GMP
The primary goal of GMP is to guarantee product safety and efficacy. Compliance with GMP standards helps prevent errors and contamination throughout the manufacturing process.
Inspections and Certification
Facilities must undergo inspections to verify compliance with GMP standards. Successful inspections lead to certification, confirming that the facility meets industry regulations and quality benchmarks.
What is the FDA classification of clean rooms?

1. Overview of FDA Classification: The FDA classifies clean rooms based on their intended use, focusing on contamination control in pharmaceutical manufacturing. These classifications ensure environments meet strict cleanliness standards.
2. Current Good Manufacturing Practices: While the FDA does not use a specific classification system like ISO, it requires adherence to Current Good Manufacturing Practices (CGMP). CGMP ensures necessary cleanliness levels for drug production.
What is a ISO 7 cleanroom?

Definition of ISO 7
A ISO 7 cleanroom is defined by ISO standards as having a maximum allowable particle count of 352,000 particles per cubic meter for particles 0.5 micrometers and larger.
Suitable Applications
ISO 7 Cleanrooms are essential in industries like pharmaceuticals and biotechnology, where moderate contamination control is necessary to ensure product quality and safety during manufacturing.
Design Requirements
These cleanrooms must incorporate efficient air filtration systems, typically using HEPA filters, to maintain required air quality levels. Regular monitoring is crucial for compliance.
Operational Procedures
Strict operational protocols must be followed in ISO 7 cleanrooms, including gowning procedures and equipment sterilization, to minimize contamination risks and maintain cleanliness.
What is a ISO 8 Cleanroom?

Definition of ISO 8
A ISO 8 cleanroom, according to ISO standards, allows for a maximum of 3,520,000 particles per cubic meter for particles 0.5 micrometers and larger, indicating lower cleanliness levels.
Appropriate Applications
ISO 8 cleanrooms are suitable for less critical operations, such as certain pharmaceutical processes and food manufacturing, where higher levels of contamination can be tolerated.
Design Considerations
These cleanrooms require basic air filtration systems, often using HEPA filters, but with less stringent requirements compared to higher-class cleanrooms. Regular maintenance remains essential.
Operational Guidelines
Personnel in ISO 8 cleanrooms must adhere to standard gowning and hygiene practices to limit contamination. However, the protocols are typically less rigorous than those in ISO 7 or higher.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU