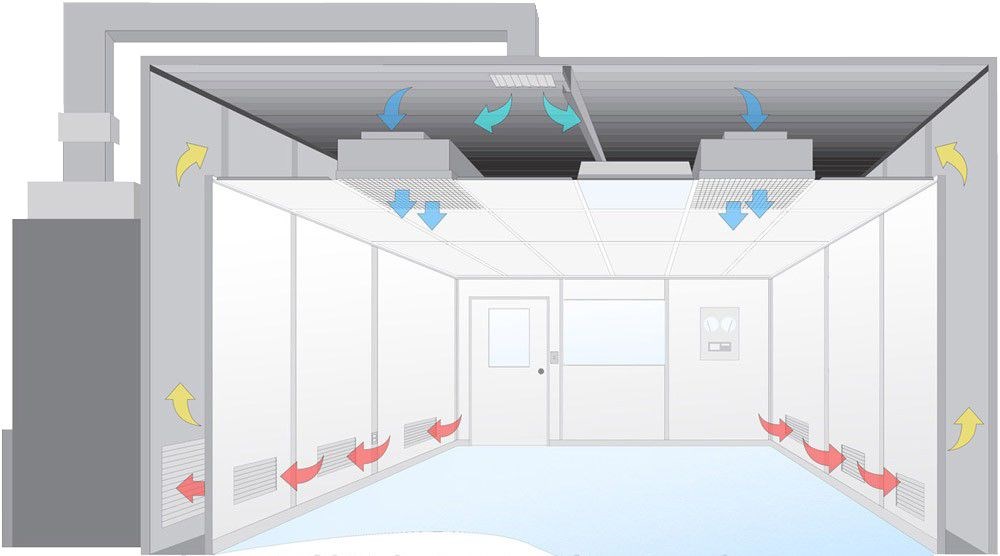
A clean room environment is a controlled space designed to minimize contamination from airborne particles, microbes, chemical vapors, or temperature fluctuations. These specialized rooms are critical in industries where even microscopic contaminants can compromise product quality, safety, or research outcomes. Clean rooms are classified based on the number and size of particles permitted per cubic meter, with adherence to international standards such as ISO 14644-1 and legacy guidelines like U.S. Federal Standard 209E.

Core Components of a Clean Room
To achieve stringent contamination control, clean rooms rely on integrated systems and protocols. Key elements include:
Air Filtration Systems: High-efficiency particulate air (HEPA) or ultra-low penetration air (ULPA) filters remove 99.97%–99.999% of particles ≥0.3 µm.
Pressure Control: Positive or negative air pressure prevents cross-contamination between adjacent areas.
Material Surfaces: Non-shedding, anti-static materials (e.g., stainless steel, epoxy-coated walls) reduce particle generation.
Garment Protocols: Operators wear gowns, gloves, and masks to limit human-borne contaminants.
Core Component Description Air Filtration System Utilizes HEPA or ULPA filters to remove airborne particles and contaminants from the air. Temperature Control Maintains specific temperature ranges to ensure product stability and prevent contamination. Humidity Control Regulates humidity levels to minimize the risk of microbial growth and maintain product integrity. Controlled Access Limits entry to authorized personnel to reduce contamination risks. Gowning Procedures Enforces strict protocols for personnel dress, including protective clothing, masks, and gloves. Clean Room Monitoring Utilizes sensors to continuously monitor particle counts, temperature, and humidity levels. Surface Materials Uses non-porous, easily cleanable materials to minimize particle accumulation and facilitate cleaning. Clean Room Protocols Establishes standard operating procedures for cleaning, maintenance, and operational practices. Validation and Testing Conducts regular testing and validation of clean room conditions to ensure compliance with standards. Emergency Equipment Provides equipment such as emergency showers and eyewashes to ensure safety in case of contamination.
These components are calibrated to meet industry-specific needs. For example, semiconductor manufacturing requires stricter particle control than pharmaceutical packaging.
Clean room environmental parameters standards
Cleanliness:
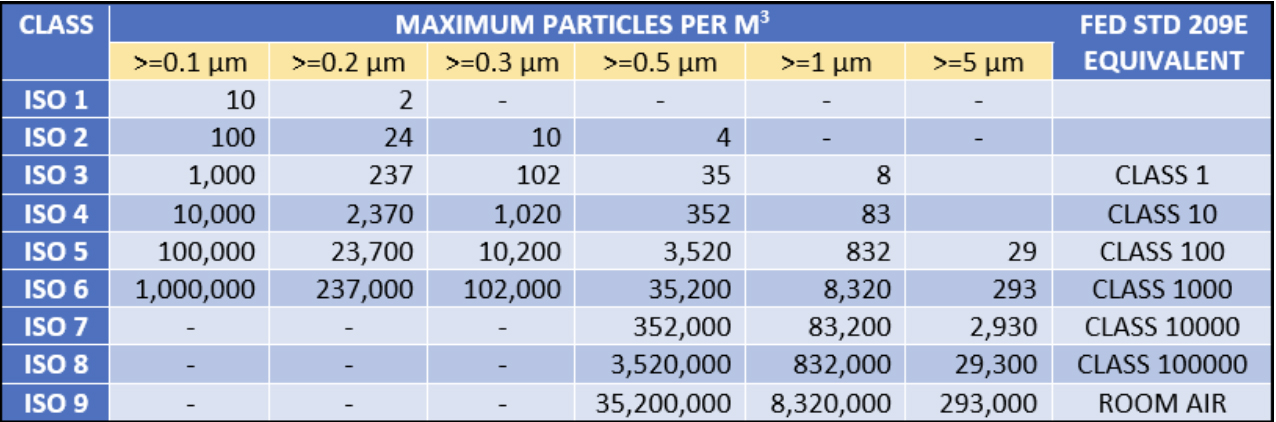
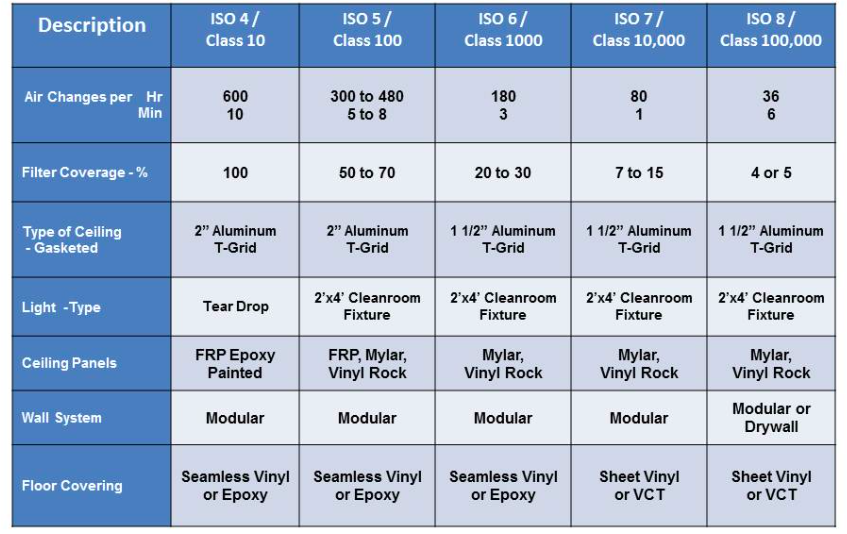
Clean room cleanliness is classified based on the concentration of airborne particles. Standards such as ISO 14644 define various cleanliness classes, from ISO Class 1 (the cleanest) to ISO Class 9, specifying the maximum allowable particle counts per cubic meter.
Temperature:
Temperature control is critical in clean rooms to ensure product stability and prevent contamination. Standards typically require maintaining specific temperature ranges, often between 20°C to 25°C (68°F to 77°F), depending on the application.
Humidity:
Humidity levels must be controlled to minimize microbial growth and maintain product integrity. The acceptable range is usually between 30% to 60% relative humidity, but this can vary based on the specific requirements of the clean room.
Pressure Differentials:
Maintaining appropriate pressure differentials between the clean room and adjacent areas is essential to prevent the ingress of contaminants. Clean rooms are typically kept at a positive pressure relative to surrounding spaces, helping to ensure that airflow is directed outwards.
Illumination:
Adequate lighting is crucial for maintaining safety and productivity in clean rooms. Standards often specify illumination levels, typically measured in lux, to ensure that work areas are well-lit without compromising cleanliness.
Noise Levels:
Noise control is important to maintain a conducive working environment. Clean room standards may include limits on noise levels, typically measured in decibels (dB), to minimize distractions and ensure effective communication among personnel.
These standards are essential for ensuring that clean rooms operate effectively, maintaining the quality and safety of products manufactured in controlled environments.
Applications of Clean Room Environments
Clean room environments are indispensable in industries where precision, sterility, and contamination control are critical. Below is an in-depth exploration of their primary applications, supported by relevant international standards and examples of how Deiiang’s solutions address these needs.
1. Pharmaceuticals and Biotechnology
The pharmaceutical sector relies on clean rooms to ensure the safety and efficacy of products. Key applications include:
Sterile drug manufacturing: Production of injectables, vaccines, and biologics requiring ISO Class 5–8 environments.
Aseptic filling: Compliance with EU Annex 1 and FDA cGMP guidelines for particulate and microbial limits.
Gene therapy and cell culture: Handling sensitive biological materials in ISO Class 7 Clean rooms with laminar airflow.
Deiiang’s modular clean rooms integrate HEPA filtration and real-time monitoring systems, aligning with WHO GMP and PIC/S standards to ensure regulatory compliance.
2. Semiconductor and Electronics Manufacturing
Microelectronics demand ultra-clean environments to prevent defects in nanoscale components:
Semiconductor fabrication: Conducted in ISO Class 1–3 clean rooms to minimize sub-0.1 µm particles.
Microchip assembly: Protection against electrostatic discharge (ESD) using anti-static flooring and ionization systems.
Flat-panel display production: Controlled humidity (<40%) to avoid moisture-induced defects.
Deiiang provides ESD-safe clean rooms compliant with iso 14644-1 and SEMI S2/S8 standards, featuring ULPA filters and temperature stability within ±0.5°C.
3. Healthcare and Medical Devices
Clean rooms are vital for both clinical and manufacturing settings:
Surgical suites: ISO Class 7 environments for infection-sensitive procedures like orthopedic implants.
Medical device sterilization: Packaging under ISO Class 5 conditions to meet ISO 13485 requirements.
Diagnostic reagent production: Ensuring reagent purity in controlled environments.
Deiiang’s healthcare-focused clean rooms feature seamless epoxy floors and automated airlock systems, reducing contamination risks by 90%.
4. Aerospace and Optics
High-precision industries use clean rooms to protect sensitive equipment:
Satellite assembly: ISO Class 6–8 rooms prevent particulate interference with optical sensors.
Laser component manufacturing: Controlled environments eliminate dust-induced laser scattering.
Composite material curing: Temperature and humidity stability per NASA STD 8719.9.
Deiiang’s aerospace-grade clean rooms utilize positive pressure control and vibration-dampened structures, meeting ECSS-Q-ST-70-01C specifications.
5. Food and Nanotechnology Research
Emerging sectors increasingly adopt clean room protocols:
Nanomaterial synthesis: ISO Class 4–6 environments for graphene and quantum dot production.
Packaged food manufacturing: Reducing microbial contamination to comply with ISO 22000.
Lab-grown meat: Cell cultivation in bio-safe clean rooms adhering to FDA Food Safety Modernization Act (FSMA).
Deiiang offers customizable solutions for niche applications, including antimicrobial wall coatings validated to ISO 20743.
Deiiang’s Role in Advancing Clean Room Technology
Deiiang supports these industries through innovation:
Smart monitoring: IoT sensors track particulate counts, temperature, and pressure differentials in real time.
Energy efficiency: Proprietary FFU (Fan Filter Unit) systems reduce energy consumption by 30% versus traditional designs.
Rapid deployment: Pre-certified modular units achieve ISO 14644 compliance within 4–6 weeks.
For example, Deiiang’s partnership with a leading vaccine manufacturer enabled the construction of 20 ISO Class 7 Clean rooms in 3 months, accelerating global vaccine distribution.
Cleanroom International Standards and Compliance Requirements

Clean room environments are governed by a complex framework of international standards and industry-specific regulations to ensure contamination control, operational safety, and product integrity. Below is a comprehensive overview of key standards, compliance criteria, and their applications across industries.
1. ISO 14644 Series: The Global Benchmark
The ISO 14644 series, developed by the International Organization for Standardization (ISO), is the most widely recognized standard for Clean Room Classification and management. Key components include:
ISO 14644-1: Defines air cleanliness classes (ISO Class 1–9) based on permissible airborne particle concentrations. For example:
ISO Class 5: ≤3,520 particles ≥0.5 µm/m³ 211.
ISO Class 9: Comparable to ordinary indoor air (≈35,200,000 particles ≥0.5 µm/m³) 7.
iso 14644-2: Specifies testing methods for compliance, including particle count, airflow velocity, and pressure differentials 2.
ISO 14644-3–7: Cover design, operation, and specialized requirements like microbial control (ISO 14698) 27.
Applications:
Semiconductor manufacturing (ISO Class 1–3) 58.
Pharmaceutical sterile production (ISO Class 5–8) 89.
2. Industry-Specific Standards
Different sectors enforce tailored regulations to address unique contamination risks:
A. Pharmaceutical and Biotechnology
GMP (Good Manufacturing Practice): Mandates air quality, microbial limits, and process controls. For example:
EU GMP Annex 1: Requires dynamic monitoring (during production) for Grade A/B areas 813.
FDA 21 CFR Part 211: Specifies clean room design and validation for drug manufacturing 7.
B. Electronics and Semiconductor
SEMI Standards: SEMI S2/S8 guidelines focus on chemical safety and electrostatic discharge (ESD) control 8.
IEST-RP-CC001: Provides protocols for HEPA/ULPA filter testing 13.
C. Healthcare and Medical Devices
ISO 13485: Requires clean room validation for sterile medical device packaging 9.
USP <797>: Governs compounding pharmacies, emphasizing airborne particulate and microbial limits 7.
3. Key Compliance Requirements
To meet international standards, clean rooms must address four critical pillars:
Air Handling Systems
Filtration: HEPA (99.97% efficiency for ≥0.3 µm particles) or ULPA (99.999% for ≥0.12 µm) filters 13.
Pressure Control: Maintain positive/negative pressure differentials (≥5–15 Pa) to prevent cross-contamination 813.
Airflow Patterns: Unidirectional (laminar) or turbulent flow, depending on ISO class 13.
Structural and Material Integrity
Non-shedding Surfaces: Stainless steel, epoxy-coated walls, and anti-static flooring 313.
Sealed Construction: Minimize gaps and joints; use airtight doors and pass-through chambers 813.
Monitoring and Validation
Particle Counting: Laser-based sensors for real-time ISO class verification 913.
Microbial Sampling: Active air sampling and surface swabs for biocontamination checks 79.
Filter Leak Testing: Use aerosol photometers to detect HEPA/ULPA breaches 9.
Operational Protocols
Garment Requirements: Full-body suits, gloves, and masks to reduce human-borne contaminants 213.
Decontamination Cycles: Routine cleaning with sporicidal agents and vaporized hydrogen peroxide (VHP) 7.
4. Regional and Legacy Standards
While ISO 14644 dominates globally, some regions or industries retain older frameworks:
U.S. Federal Standard 209E: Historically defined classes as "Class 100" or "Class 10,000" (particles per cubic foot). Though obsolete, it remains influential in certain sectors 511.
China’s GB 50073/GB 50472: Align with ISO 14644 but include additional HVAC and noise limits (e.g., ≤65 dB(A) for non-unidirectional airflow) 811.
EU GMP Annex 1: Stricter than ISO, requiring continuous particle monitoring in Grade A zones 8.
5. Emerging Trends and Innovations
Smart Clean Rooms: IoT-enabled sensors for real-time environmental tracking (e.g., Deiiang’s modular systems with predictive analytics) 13.
Sustainability: Energy-efficient HVAC designs and recyclable materials to meet net-zero goals 8.
Automation: Robotics for reduced human intervention in high-risk processes 8.
Summarize
The maintenance of the clean room environment is a complex and systematic process involving the control and management of multiple technical parameters such as cleanliness, temperature, humidity, and noise. Through regular monitoring, scientific management, and personnel training, it can effectively ensure that the environmental quality of the clean room meets industry standards and production requirements.
In the context of the rapid development of science and technology, the requirements for clean room environment are also constantly increasing. In the future, more attention will be paid to the application of intelligent and automated management methods to improve the operating efficiency and maintenance level of clean rooms. Through continuous innovation and improvement, clean rooms will provide higher quality production and experimental environments for various industries, and escort scientific and technological progress and product safety.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU