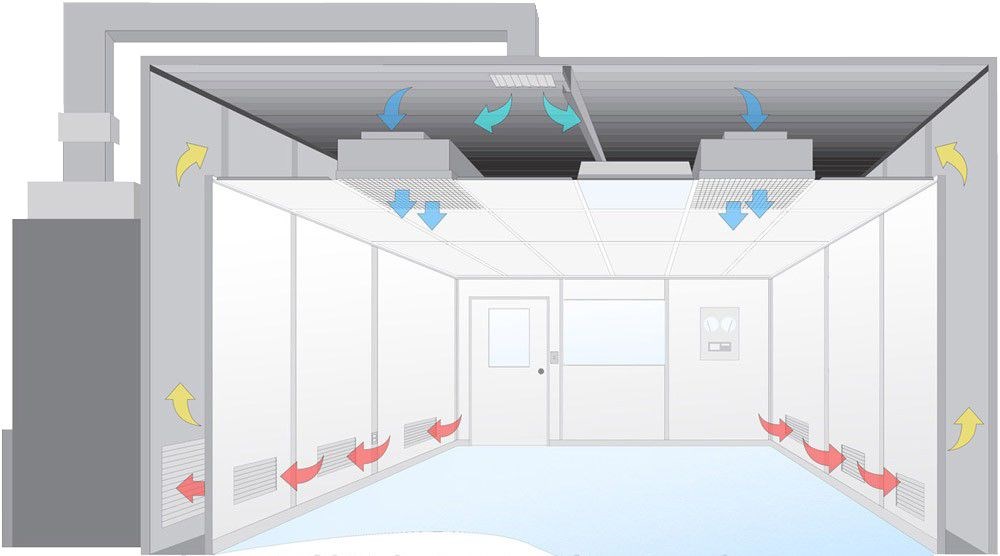
The design of pharmaceutical clean rooms is critical to ensuring that drug manufacturing processes meet stringent Good Manufacturing Practice (GMP) standards. These controlled environments are essential for product safety, efficacy, and regulatory compliance. This article explores the key components of pharma Clean room design that help achieve these high standards.

What is a PharmA Clean Room?
A pharma clean room is a controlled environment where particulate and microbial contamination is regulated to meet specific cleanliness levels. These rooms are essential for industries like biotechnology and pharmaceuticals, where maintaining the integrity of products is of utmost importance.
Clean rooms are classified based on air cleanliness standards like those defined in ISO 14644, with GMP guidelines further ensuring that environmental conditions support pharmaceutical manufacturing processes.
Core Elements of Pharmaceutical Clean Room Design
1. Personnel Access Areas
Effective design includes change rooms, shoe-changing areas, handwashing and disinfection areas, buffer rooms, and clean corridors. These are vital for minimizing contamination risk when personnel enter and exit the controlled environment.
2. Material Transfer Areas
Design considerations include raw material warehouses, finished product storage, unpacking rooms, temporary storage, and buffer zones. Efficient material flow minimizes cross-contamination and supports a seamless supply chain.
3. Production Zones
Production areas such as preparation, filling, and packaging rooms must be meticulously planned to support regulated processes and optimize operational flow.
4. Auxiliary Areas
Support areas like cleaning equipment rooms, tool rooms, pure water rooms, and equipment rooms are crucial for maintaining the cleanliness and efficiency of the primary production zones.

GMP Standards for Pharmaceutical Clean Rooms
GMP Standards govern pharmaceutical clean rooms' operation, emphasizing contamination control, quality assurance, and operational consistency.
Regular audits and validations, rigorous documentation, and strict environmental monitoring are integral to maintaining compliance. The standards specify precise cleanliness levels and environmental conditions required for each manufacturing stage.
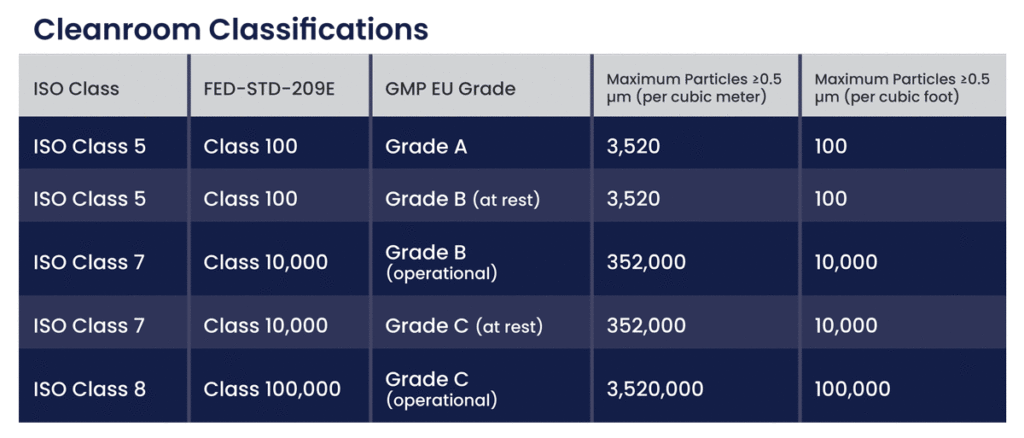
GMP Pharmaceutical Clean Room Design Process
Designing a clean room involves a meticulous process aligned with GMP standards.
Planning Phase: Identify the clean room requirements, including the necessary iso class and GMP compliance aspects. This phase involves engaging stakeholders and consulting with experts like Deiiang™, who provide tailored design solutions.
Design and Construction: Develop detailed Layout Designs that incorporate all functional and auxiliary areas. Construction should use compliant materials and uphold the highest hygiene standards.
Validation and Commissioning: Conduct thorough testing and validation to confirm that the clean room meets all regulatory standards. This includes airflow testing, particulate measurements, and Environmental monitoring to ensure that operational parameters align with GMP requirements.
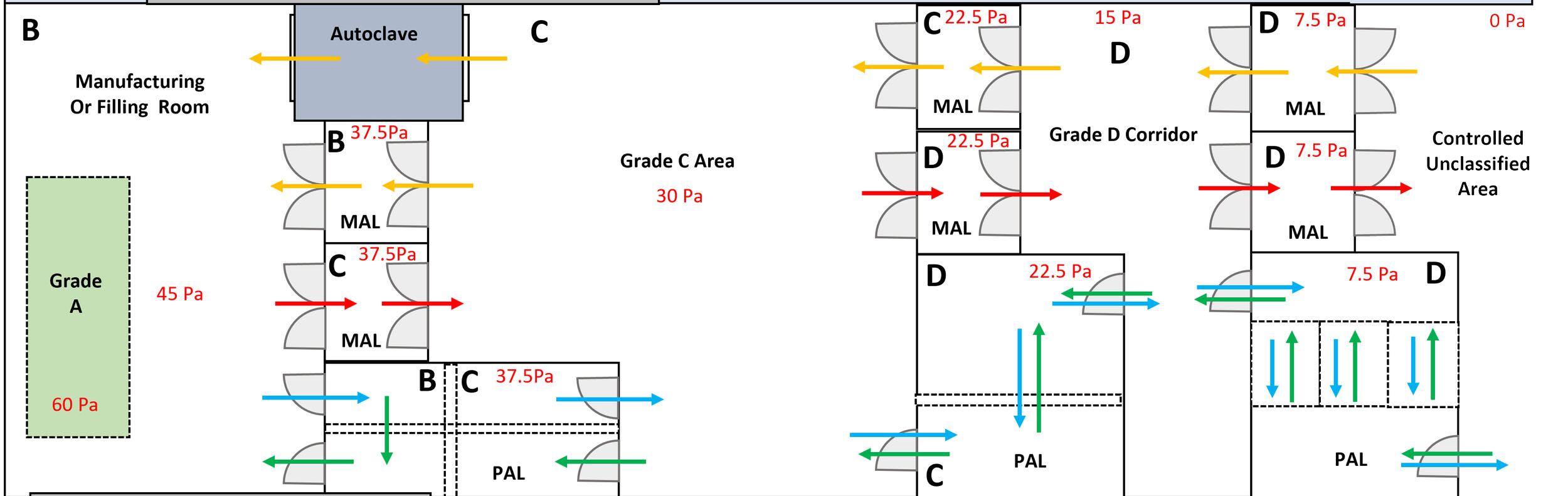
Conclusion
Designing a pharma clean room that meets GMP standards is a comprehensive process requiring attention to detail and an unwavering commitment to quality. By integrating core design elements and following a structured design and validation process, pharmaceutical companies can ensure their facilities maintain product integrity and regulatory compliance. Partnering with industry leaders like GCC®️ and Deii®️ ensures that clean room designs are forward-thinking and aligned with the latest advancements in contamination control and process optimization.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU