5 Key Components of Good Manufacturing Practices
Good Manufacturing Practices (GMP) are essential for ensuring the consistent quality and safety of products in various industries, particularly pharmaceuticals, food, and cosmetics. Understanding the key components of GMP can help organizations maintain compliance and enhance product quality.
The 5 Critical Components of GMP
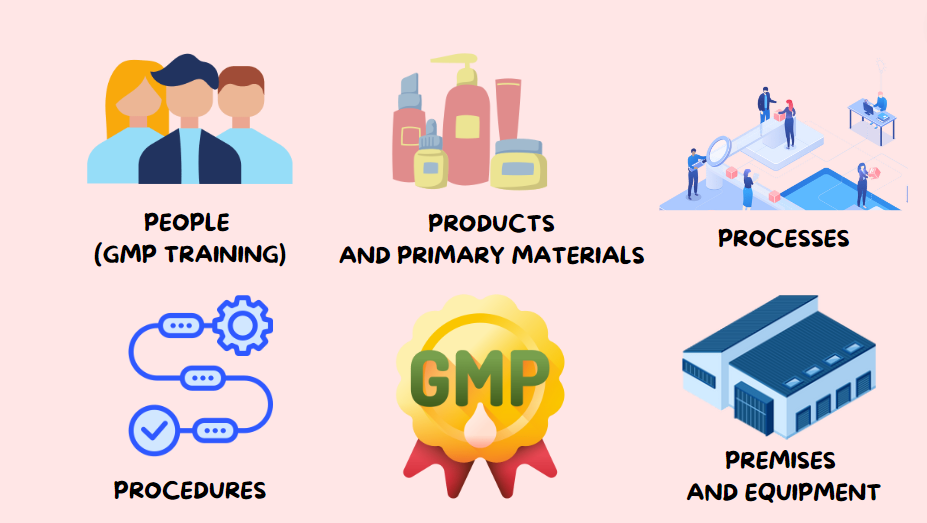
1. Quality Management System (QMS)
A robust Quality Management System (QMS) is fundamental to GMP. It encompasses organizational structure, responsibilities, procedures, processes, and resources for implementing quality management.
Standard: ISO 9001:2015
2. Personnel Training and Qualification
Qualified personnel are crucial in gmp environments. Regular training ensures that employees understand their roles and the importance of following GMP protocols.
Recommendation: Annual training sessions (FDA)
3. Facility and Equipment Maintenance
The design, location, and maintenance of facilities and equipment directly impact product quality. GMP requires that facilities be designed to prevent contamination.
Standard: ISO 13485 for medical devices
4. Document Control and Record Keeping
Effective document control is essential for maintaining compliance with GMP. All procedures, specifications, and quality records must be documented.
Requirement: FDA 21 CFR Part 211
5. Quality Control and Testing
Quality control (QC) ensures products meet established quality standards before reACHing consumers. This involves systematic sampling, testing, and validation.
Guideline: ICH Q7 for Active Pharmaceutical Ingredients
GMP-compliant manufacturing facility
Standard Operating Procedures (SOPs) for GMP
Definition of SOP
A Standard Operating Procedure (SOP) is a documented process that outlines the steps necessary to ensure compliance with Good Manufacturing Practices (GMP). It serves as a guideline for consistent operations.
Importance of SOPs
SOPs are crucial for maintaining quality and safety in manufacturing. They provide clear instructions, minimize variations, and help ensure that all employees follow the same procedures to meet regulatory standards.
Components of SOPs
An effective SOP includes objectives, scope, responsibilities, procedures, and references. It should be clear, concise, and easily accessible to all personnel involved in the manufacturing process.
The Golden Rules of GMP
Rule 1: Quality Assurance
Systematic checks and balances to meet regulatory and safety standards consistently.
Rule 2: Personnel Training
Employees must understand GMP principles to reduce contamination and errors.
Rule 3: Documentation
Records should be maintained for all processes to ensure traceability.
Rule 4: Facility Maintenance
Maintain clean and organized manufacturing environments.
Rule 5: Equipment Calibration
Regular calibration ensures accurate measurements and operations.
Rule 6: Risk Management
Identify, assess, and mitigate manufacturing risks.
The Four Domains of GMP
| Domain | Description |
|---|---|
| Quality Management | Ensures products meet set standards through quality planning and continuous improvement. |
| Production | Focuses on manufacturing processes to maintain product integrity and safety. |
| Quality Control | Testing and monitoring of materials and finished products. |
| Personnel | Emphasizes qualified and trained staff for GMP compliance. |
Relevant Standards and Regulations
- ISO 9001:2015 - Quality Management Systems
- ISO 13485 - Quality Management for Medical Devices
- FDA 21 CFR Part 211 - Current Good Manufacturing Practice for Finished Pharmaceuticals
- ICH Q7 - Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients
- WHO Good Manufacturing Practices - Guidelines for Pharmaceutical Production
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



