Cleanroom certification is an important step to ensure that laboratories, production workshops, and other critical environments meet specific cleanliness standards. The certification process not only helps maintain product quality, but also enhances the company's reputation. The following is a clear process for modular cleanroom certification. It is divided into major steps, and eACH step has specific sub-steps.
Part I: Understanding Cleanroom Certification Types
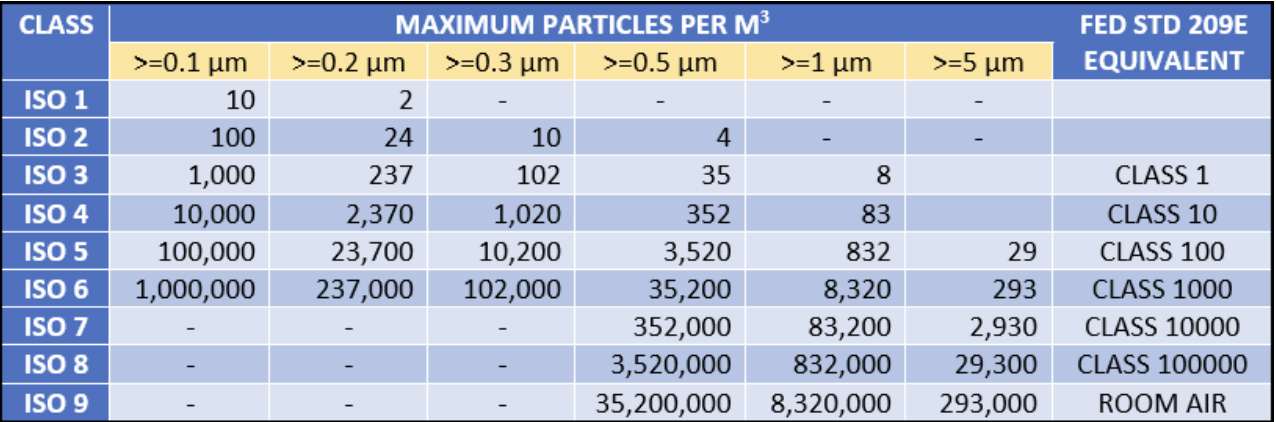
1. iso 14644 Standard
The iso 14644 series of standards is the most widely adopted cleanroom standard internationally. This standard divides cleanrooms into multiple levels, from ISO1 (cleanest) to ISO9 (dirtier). For example, iso5 requires no more than 3,520 particles per cubic meter of air, which is suitable for demanding industries such as semiconductor and pharmaceutical production.
2. GMP Certification
Good Manufacturing Practice (GMP) certification is essential for pharmaceutical and biologics manufacturers. GMP requires that the design, management, and operation of cleanrooms meet strict hygiene standards to ensure that products are not contaminated. For example, GMP has clear regulations on cleanroom temperature and humidity, cleanliness, and personnel operations.
3. Other industry standards
Different industries may have specific certification requirements, such as aerospace, electronics manufacturing, and other fields. Understanding these industry standards is essential to ensure that cleanrooms meet specific business needs. Certification in different fields may involve unique testing methods and standards, such as AS9100 (aerospace) or ISO/IEC 17025 (laboratory management).

Part II: Evaluate existing facilities
1. Preliminary audit
Before starting certification, a preliminary audit of the existing cleanroom facilities is required. This includes checking the room layout, air circulation, material use, etc. Through the assessment, it is understood whether the current facilities meet the required cleanliness standards, and usually there is room for improvement.
2. Air quality monitoring
Detailed monitoring of the air quality of the cleanroom is an important part of the assessment. Use a particle counter to detect the number of particles in the air to ensure that it is within the standard range. For example, the requirement for ISO7 is no more than 352,000 particles per cubic meter. This monitoring can help determine the current status of the facility and areas for improvement.
3. Identify potential risks
The assessment process also needs to identify potential sources of contamination, such as personnel entry and exit, equipment use, and material storage. These risk factors will directly affect the cleanliness of the cleanroom, so they should be taken seriously and addressed in the improvement plan.

Part III: Correction and Optimization
1. Upgrade the air filtration system
Once the problem is identified, the cleanroom needs to be corrected. Upgrading the air filtration system is a key step, usually using HEPA or ULPA filters. These filters can effectively remove tiny particles to ensure that air quality meets standards. For example, HEPA filters can be as efficient as 99.97% and can capture particles with a diameter of 0.3 microns.
2. Improve workflow
It is equally important to optimize the workflow. Establish strict operating procedures to ensure that the behavior of personnel in the cleanroom does not cause contamination. For example, all personnel entering the cleanroom must wear special clean clothes, gloves and masks, and disinfect their hands before entering.
3. Regular maintenance and monitoring
After rectification, the cleanroom should be maintained and monitored regularly. This includes regular testing of air quality, equipment operating status and cleanliness. Establishing a complete record system will help track the status of the cleanroom and ensure that it always meets the certification standards.

Part 4: Conducting tests and applying for certification
1. Choosing a professional testing agency
After the rectification is completed, a third-party testing agency with certification qualifications needs to be selected for testing. These agencies usually conduct a comprehensive assessment based on ISO standards. The report will include results such as air cleanliness, temperature and humidity, and microbial tests to ensure that the modular cleanroom meets the relevant standards.
2. Submitting a certification application
After passing the test, the next step is to submit an application to the relevant certification body. During the application process, relevant test reports and rectification records need to be prepared to prove that the cleanroom meets the certification requirements. Usually, the certification body will conduct a preliminary review of the submitted materials and arrange an on-site inspection.
3. On-site audit and certificate issuance
The certification body will conduct an on-site audit to evaluate the actual operating status of the cleanroom. If the audit is qualified, a certification certificate will be issued. This certificate is usually valid for one year or longer, depending on the type of certification and the requirements of the organization. After the certificate is issued, the company needs to maintain the management standards of the cleanroom to ensure continued compliance with the certification requirements.

Part 5: Maintaining and renewing certification
1. Continuous monitoring and maintenance
In order to ensure that the cleanroom continues to meet the certification standards, the company needs to establish a continuous monitoring and maintenance plan. This includes regular testing of air quality and cleanliness, recording and analyzing monitoring data, and ensuring that problems are discovered and adjusted in a timely manner. It is generally recommended to conduct air quality monitoring once a month to ensure that the cleanroom standards are always met.
2. Employee training and management
Employee training and management are important aspects of maintaining cleanroom certification. Regularly train employees on cleanroom operations to ensure that they understand how to reduce contamination risks and follow cleanroom operating procedures. Studies have shown that proper training can reduce the risk of contamination caused by human error by more than 30%, significantly improving the cleanliness of the cleanroom.
3. Regular review and recertification
Most certification bodies require companies to review and recertify before the end of the certificate validity period. Companies need to prepare in advance to ensure that all facilities and operations meet the latest standards and requirements. Re-audits usually include on-site inspections, document reviews, and retesting to ensure that companies always maintain high standards of cleanroom environments.

List of industry-related standards and specifications
In the process of cleanroom certification, it is very necessary to understand the relevant industry standards. The following are some common cleanroom-related standards and specifications:
ISO 14644: Cleanliness standards for cleanrooms and related controlled environments
GMP: Good manufacturing practices for pharmaceutical production
ISO/IEC 17025: Laboratory management system standards
AS9100: Quality management standards for the aerospace industry
ISO 9001: Quality management system standards
FDA 21 CFR Part 210/211: U.S. Food and Drug Administration regulatory requirements for pharmaceutical manufacturing
By following these standards, companies can ensure that the design, operation, and management of their cleanrooms meet industry best practices, thereby improving product quality and consumer trust. Clean room certification is not only a compliance requirement, but also an important manifestation of a company's pursuit of excellence and continuous improvement.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



