
In pharmaceutical, biotechnology, and medical device industries, how do you ensure your cleanroom is not just "clean" but fully compliant? GMP cleanroom requirements form the foundation of quality manufacturing. Good Manufacturing Practice (GMP) represents a system that ensures products are consistently produced and controlled according to quality standards. These GMP cleanroom requirements are critical for protecting product quality, patient safety, and regulatory compliance.
This comprehensive guide from Deiiang™ will walk you through everything from fundamental concepts to practical implementation of GMP cleanroom requirements. You'll learn about international standards, classification systems, design considerations, validation processes, and ongoing operational requirements. Understanding these GMP cleanroom requirements is essential for any facility seeking regulatory approval and market access.
GMP Cleanroom Fundamentals: Concepts & Necessity
Understanding the fundamental concepts behind GMP cleanroom requirements is the first step toward compliance. These requirements aren't arbitrary—they're scientifically grounded in contamination control principles. Proper implementation of GMP cleanroom requirements prevents product contamination, cross-contamination, and ensures consistent quality.
Meeting GMP cleanroom requirements involves more than just particle counts; it encompasses the entire quality management system. According to Deiiang™ experts, facilities that thoroughly understand these GMP cleanroom requirements experience 60% fewer regulatory observations and achieve compliance 40% faster than those who approach them as a checklist.
What is Good Manufacturing Practice (GMP)?
Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards. It's designed to minimize the risks involved in pharmaceutical production that cannot be eliminated through testing the final product. The core GMP principles, often called the 5Ps, include:
- People: All personnel must be adequately trained and follow procedures
- Premises: Facilities must be designed, maintained, and validated appropriately
- Processes: Manufacturing processes must be clearly defined and controlled
- Products: All materials and products must meet specifications
- Procedures: Instructions must be written in clear, unambiguous language
Statistics show that GMP non-compliance costs the pharmaceutical industry approximately $3-5 billion annually in recalls, fines, and warning letters. As Jason.peng, Product Designer at Deiiang™ notes, "A proactive approach to GMP cleanroom requirements saves significant costs compared to reactive compliance measures."
What is a Cleanroom?
A cleanroom is a controlled environment where pollutants like dust, airborne microbes, and aerosol particles are filtered out to provide the cleanest area possible. Cleanrooms are classified according to the number and size of particles permitted per volume of air. Stringent GMP cleanroom requirements govern not just airborne particles but also temperature, humidity, air pressure, and airflow patterns.
Why Does GMP Require Cleanrooms?
GMP requires cleanrooms because they serve as critical physical barriers against contamination. In pharmaceutical manufacturing, contamination can compromise product safety and efficacy, potentially harming patients. Cleanrooms provide the controlled environment necessary to prevent microbial growth, cross-contamination, and particulate contamination during manufacturing processes.

GMP Cleanroom Classification & International Standards
Understanding cleanroom classification systems is fundamental to meeting GMP cleanroom requirements. Different manufacturing activities require different levels of environmental control, and classification systems provide the framework for specifying these GMP cleanroom requirements. Proper classification ensures that the cleanroom environment is appropriate for the specific manufacturing activities conducted within it.
Global harmonization of GMP cleanroom requirements continues to evolve, with recent updates to major guidelines emphasizing risk-based approaches and contamination control strategies. Facilities designed by Deiiang™ incorporate these updated GMP cleanroom requirements, resulting in 30% more efficient compliance with international standards.
GMP cleanroom grade Classification (A, B, C, D Zones)
The EU GMP Annex 1 and WHO GMP guidelines classify cleanrooms into four grades: A, B, C, and D. Grade A represents the highest level of cleanliness, typically used for high-risk operations like aseptic filling. Understanding these classifications is essential for proper implementation of GMP cleanroom requirements.
| Grade | ISO 14644-1 Classification | Maximum Particles/m³ ≥0.5µm | Maximum Particles/m³ ≥5µm | Typical Applications |
|---|---|---|---|---|
| A | ISO 5 (at rest and in operation) | 3,520 | 20 | Aseptic filling, open container operations |
| B | ISO 6 (at rest), ISO 7 (in operation) | 3,5200 (at rest) | 29 (at rest) | Background for grade A zones, aseptic preparation |
| C | ISO 7 (at rest), iso 8 (in operation) | 352,000 (at rest) | 2,930 (at rest) | Less critical stages of sterile manufacturing |
| D | ISO 8 (at rest) | 3,520,000 (at rest) | 29,300 (at rest) | Handling of components, washing equipment |
Major International Regulations & Guidelines
Several international regulations govern GMP cleanroom requirements. Key among them are:
- EU GMP Annex 1 (2022): The latest version emphasizes contamination control strategy and risk-based approaches to sterile manufacturing
- FDA cGMP (21 CFR Part 210/211/820): Critical for market access in the United States
- WHO GMP: Particularly important for manufacturers targeting global markets, especially developing countries
- ISO 14644 Series: Provides the technical framework for cleanroom classification and testing
According to Jason.peng, "A Deiiang™ cleanroom designed to meet the updated EU GMP Annex 1 requirements typically incorporates 15-20% more monitoring points and 25% more robust contamination control measures compared to previous standards."
GMP Cleanroom Design Requirements
Proper design is foundational to meeting GMP cleanroom requirements. The design phase establishes the framework for all subsequent validation, operation, and maintenance activities. Effective implementation of GMP cleanroom requirements during design prevents costly modifications later and ensures long-term compliance.
Deiiang™ employs a systematic approach to GMP cleanroom requirements during design, incorporating computational fluid dynamics (CFD) modeling to predict airflow patterns and contamination risks. This approach has been shown to reduce design-related compliance issues by up to 70% compared to traditional design methods.
Facility Layout & Zoning
Proper facility layout is critical for meeting GMP cleanroom requirements. The design should incorporate unidirectional flow, physical segregation between different cleanliness zones, and appropriate pressure cascades. A typical pressure differential of 10-15 Pascals between adjacent cleanliness grades is recommended in GMP cleanroom requirements to prevent cross-contamination.
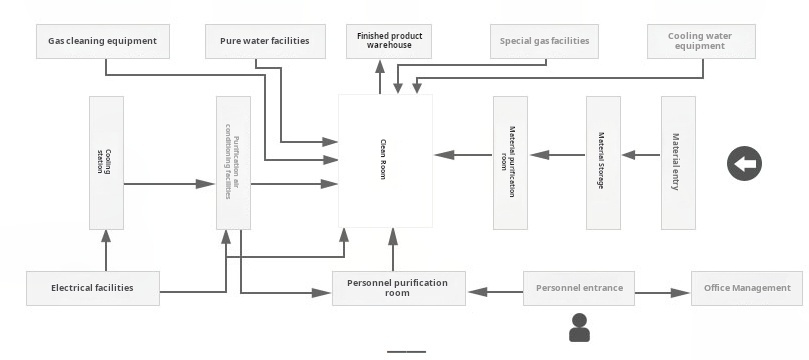
HVAC System Design
The HVAC system is the heart of any cleanroom, responsible for maintaining the environmental conditions specified in GMP cleanroom requirements. Key design considerations include:
- Air Change Rates: Grade A/B areas typically require 20-40 air changes per hour (ACH), while grade c/D may require 10-20 ACH
- Filtration: HEPA filters (99.97% efficient on 0.3µm particles) for Grades A-C; ULPA filters (99.999% efficient) for critical Grade A applications
- Pressure Control: Automated control systems to maintain required pressure differentials
The formula for calculating air changes per hour is: ACH = (Airflow rate in m³/h) / (Room volume in m³). For example, a 100m³ grade b cleanroom requiring 25 ACH needs an airflow rate of 2,500 m³/h.
Building Materials & Finishes
Materials used in GMP cleanrooms must meet specific requirements: non-shedding, easy to clean, resistant to chemicals and disinfectants, and durable. Deiiang™ typically recommends:
- Seamless epoxy or polyurethane flooring with coved bases
- Non-porous wall panels (FRP, powder-coated steel, or stainless steel)
- Suspended grid ceilings with sealed cleanroom panels
- Stainless steel fixtures and furniture

Utilities & Support Systems
All utilities serving GMP cleanrooms must be designed to prevent product contamination. Key systems include:
- Purified Water (PW) and Water for Injection (WFI): Must meet pharmacopeial standards with validated sanitization systems
- Compressed Gases: Oil-free compressors with appropriate filtration
- Clean Steam: For sterilization processes, generated from WFI-quality feedwater
GMP Cleanroom Construction, Validation & Testing
Validation provides documented evidence that a cleanroom consistently meets all specified GMP cleanroom requirements. The validation process follows a lifecycle approach, beginning with design qualification and continuing through performance qualification. Comprehensive validation is non-negotiable for facilities subject to GMP cleanroom requirements.
According to Deiiang™ validation data, facilities that implement rigorous testing protocols during initial qualification experience 45% fewer deviations during routine operations. This underscores the importance of thorough validation in meeting long-term GMP cleanroom requirements.
Construction Quality Control
Quality control during construction ensures that the built environment matches the design intent. Key aspects include:
- Verification of material certifications
- Inspection of sealing integrity at all penetrations and joints
- Confirmation of proper installation of all components
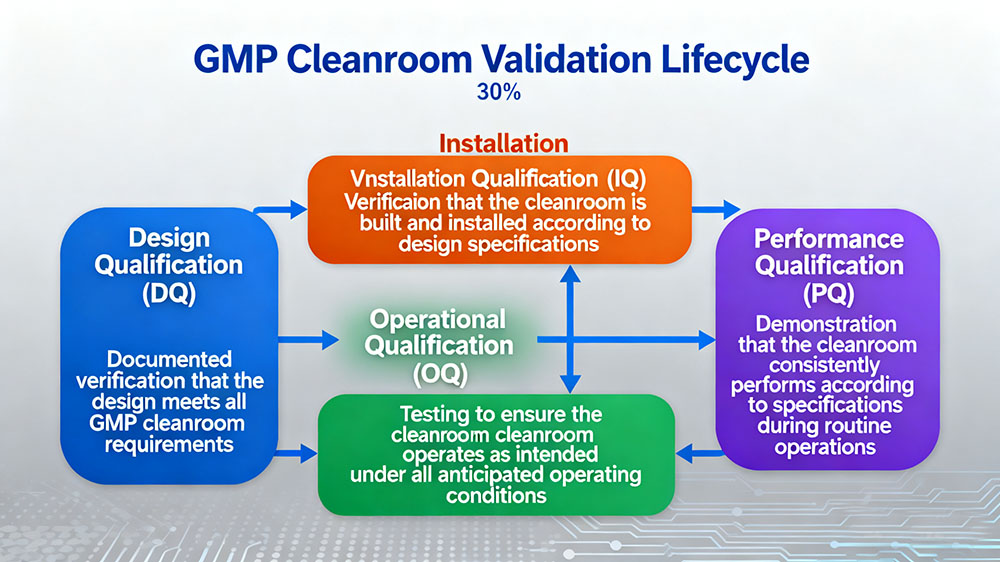
Validation Lifecycle
The validation lifecycle for GMP cleanrooms consists of four key stages:
- Design Qualification (DQ): Documented verification that the design meets all GMP cleanroom requirements
- Installation Qualification (IQ): Verification that the cleanroom is built and installed according to design specifications
- Operational Qualification (OQ): Testing to ensure the cleanroom operates as intended under all anticipated operating conditions
- Performance Qualification (PQ): Demonstration that the cleanroom consistently performs according to specifications during routine operations
Key Testing Projects & Acceptance Criteria
Comprehensive testing is essential to verify compliance with GMP cleanroom requirements. Key tests include:
| Test Parameter | Standard/Method | Frequency | Acceptance Criteria |
|---|---|---|---|
| Airborne Particle Count | ISO 14644-1 | 6-12 months | Per ISO class and GMP grade |
| Airflow Velocity/Uniformity | iso 14644-3 | 12 months | 0.45 m/s ±20% (Grade A unidirectional) |
| HEPA Filter Integrity | ISO 14644-3 | 12-24 months | ≤0.01% leak for HEPA, ≤0.005% for ULPA |
| Pressure Differential | ISO 14644-3 | Continuous monitoring | 10-15 Pa between adjacent grades |
| Microbial Monitoring | EU GMP Annex 1 | Each operational shift | Grade-dependent action limits |
Jason.peng emphasizes that "Proper testing requires specialized equipment and trained personnel. Deiiang™ validation teams use calibrated equipment traceable to national standards and follow statistically sound sampling plans to ensure representative data."
GMP Cleanroom Operation & Maintenance Requirements
Ongoing operation and maintenance are where GMP cleanroom requirements meet daily practice. Even perfectly designed and validated cleanrooms can fall out of compliance without proper operational controls. Effective implementation of GMP cleanroom requirements during operations ensures consistent product quality and regulatory compliance.
Data from Deiiang™ maintenance programs shows that facilities with comprehensive operational controls experience 60% fewer deviations and 35% lower contamination rates compared to those with reactive maintenance approaches. This demonstrates the tangible benefits of rigorous adherence to GMP cleanroom requirements.
Personnel Control & Training
Personnel represent the largest potential contamination source in cleanrooms. GMP cleanroom requirements mandate:
- Comprehensive gowning procedures with demonstrated competency
- Regular training on contamination control principles
- Health screening and exclusion policies for ill personnel
- Strict limits on the number of personnel in cleanrooms
A typical Grade A/B gowning procedure involves 8-12 separate steps and takes 10-15 minutes to complete properly.
Cleaning & Disinfection Procedures
Effective cleaning and disinfection are fundamental to meeting GMP cleanroom requirements. Key elements include:
- Validated cleaning procedures with defined frequencies
- Rotation of sporicidal agents to prevent resistance
- Environmental monitoring to verify cleaning effectiveness
- Documentation of all cleaning activities
Environmental Monitoring
Continuous environmental monitoring provides data to demonstrate ongoing compliance with GMP cleanroom requirements. A comprehensive program includes:
- Non-viable particle counting
- Viable air sampling (active and passive)
- Surface monitoring
- Personnel monitoring

Change Control & Deviation Management
Formal change control processes ensure that any modifications to facilities, equipment, or procedures don't adversely impact product quality or compliance with GMP cleanroom requirements. Similarly, deviation management systems ensure proper investigation and corrective actions when excursions occur.
Continuous Maintenance & Revalidation
GMP cleanroom requirements mandate ongoing maintenance and periodic revalidation to ensure continued compliance. Key activities include:
- Preventive maintenance of HVAC systems and equipment
- Regular calibration of monitoring instruments
- Annual review of environmental monitoring data
- Revalidation typically every 12-24 months, or after significant changes
Choosing a Qualified GMP Cleanroom Solutions Provider
Selecting the right partner is crucial for successfully implementing GMP cleanroom requirements. An experienced provider brings technical expertise, regulatory knowledge, and practical experience that can significantly impact project success and long-term compliance with GMP cleanroom requirements.
Deiiang™ has supported over 200 pharmaceutical and biotech clients in achieving and maintaining compliance with GMP cleanroom requirements. Our data shows that projects completed with qualified providers achieve regulatory approval 50% faster and experience 65% fewer compliance issues during inspections.
Why Professional Services?
GMP cleanrooms are highly complex environments requiring specialized knowledge in multiple disciplines: engineering, microbiology, regulatory affairs, and quality systems. Professional providers like Deiiang™ offer integrated solutions that address all aspects of GMP cleanroom requirements, from initial concept through ongoing compliance.
Key Evaluation Criteria
When selecting a provider for GMP cleanroom solutions, consider:
- Regulatory Experience: Track record with FDA, EMA, and other relevant authorities
- Technical Expertise: Knowledge of current standards and best practices
- Project Management: Ability to deliver on time and within budget
- Quality Systems: Documentation and validation capabilities
- References: Successful projects in similar applications
Deiiang™ GMP Cleanroom Solutions Advantage
Deiiang™ brings unique value to GMP cleanroom projects through:
- Integrated design-build-validate-maintain approach
- Proprietary design tools including advanced CFD modeling
- Comprehensive documentation and validation packages
- Ongoing support and maintenance programs
Client Success Story: A major biotech company needed to upgrade their Grade B cleanroom to meet updated EU GMP Annex 1 requirements. Deiiang™ completed the redesign, construction, and validation in just 14 weeks, achieving a 30% reduction in energy consumption while improving contamination control performance.
Frequently Asked Questions (FAQ)
Q1: What's the difference between a GMP cleanroom and a regular cleanroom?
A: While both control particulate contamination, GMP cleanrooms must comply with specific regulatory requirements covering the entire quality system, including validation, documentation, personnel training, and ongoing monitoring. Regular cleanrooms may focus only on particulate control without the comprehensive quality system requirements.
Q2: How long does it take to build a new GMP cleanroom?
A: Timeline varies based on complexity, but a typical project takes 6-18 months from design through validation. Smaller projects might complete in 4-6 months, while large, complex facilities can take 2+ years. Deiiang™ proprietary design methods can reduce timelines by 20-30% compared to conventional approaches.
Q3: Are GMP cleanrooms expensive to operate?
A: Operating costs are significant due to high energy consumption (HVAC systems), rigorous monitoring, maintenance, and personnel requirements. However, these costs are essential for product quality and regulatory compliance. Energy-efficient designs from Deiiang™ can reduce operating costs by 15-25% while maintaining compliance.
Q4: How often must GMP cleanrooms be revalidated?
A: Most regulators expect annual revalidation, though the frequency should be risk-based. Significant changes to facilities, equipment, or processes require revalidation regardless of schedule. Deiiang™ recommends continuous monitoring with formal revalidation every 12 months for high-risk areas.
Conclusion & Call to Action
Meeting GMP cleanroom requirements is a complex but essential undertaking for pharmaceutical, biotechnology, and medical device manufacturers. From proper classification and design through validation and ongoing operation, each element plays a critical role in ensuring product quality and patient safety.
By understanding and implementing these requirements comprehensively, manufacturers can achieve regulatory compliance, protect product quality, and maintain market access. The investment in proper GMP cleanroom implementation pays dividends in reduced regulatory risk, improved product quality, and enhanced business reputation.
Ready to Ensure Your Cleanroom Meets All GMP Requirements?
Contact our GMP cleanroom experts today for a comprehensive assessment of your facility
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


