1. Subject Content Purpose
This standard stipulates the basic requirements for the management of the sampling room. This procedure stipulates the basic requirements for the cleaning and sanitation of the clean room. This procedure is specially formulated.
2. Scope of application
This standard applies to all personnel entering and leaving the sampling room. This procedure applies to the cleaning management of all personnel, materials and sampling vehicles entering and leaving the clean room.

(Figure 1: Clean room hygiene)
3. Responsibilities
QC Room Director: Responsible for organizing the formulation of this procedure
Quality Department Manager: Responsible for organizing the review of this procedure
General Manager: Responsible for approving this procedure
QC Room Director: Responsible for organizing the formulation of this procedure
QA Room Director: Responsible for organizing the review of this procedure
General Manager of Quality Department: Responsible for approving this procedure
4. Content
4.1 Cleaning frequency: Clean and disinfect after eACH entry and use,
4.2 Cleaning tools: rags, cotton wool.
4.3 Cleaning agents and disinfectants: 1% detergent, 0.2% Sanisol solution, 75% ethanol.
4.4 Cleaning and disinfection methods.
4.4.1 First wipe the surface of the clean operating table, wall, door and window with a rag. If necessary, use a rag dipped in 1% detergent solution to remove the hard-to-clean dirt, and then wipe it dry with purified water.
4.4.2 Then wipe with 0.2% new chlorhexidine solution (or 75% ethanol), and finally sterilize with environmental ozone before the experiment.
4.4.3 The disinfectant is 0.2% new chlorhexidine solution and 75% ethanol alternately, and the service life is once a month.
4.5 Precautions.
4.5.1 It is strictly forbidden to clean during the experiment to avoid contamination of the production process.
4.5.2 The clean room should be self-cleaned half an hour in advance.
4.5.2 Materials and sampling utensils entering the clean room should be sterilized by ultraviolet in the buffer room.
4.5.3 Non-experimental personnel and cleaning personnel are not allowed to enter the clean room at will.
4.5.4 The clean room shall carry out daily monitoring of suspended particles and settling bacteria in accordance with the clean area.
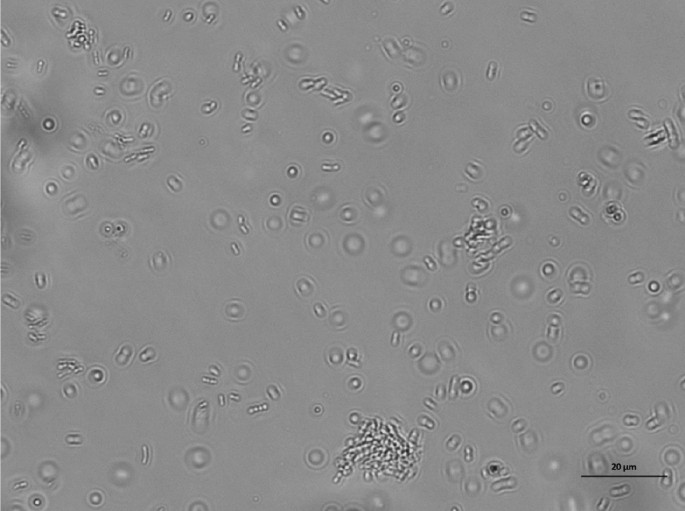
(Figure 2: Monitoring of sedimentation bacteria)
4.6 Evaluation of cleaning effect: No trace of pollution is observed visually.
4.7 Cleaning, drying and storage of cleaning tools: If there are stains on the rag, rinse it with purified water and place it in the designated position of the ware rack in the ware storage room.
Before sampling, the QC laboratory technician shall self-clean the sampling room half an hour in advance.
The materials and sampling utensils entering the sampling room shall be sterilized by ultraviolet in the buffer room.
The laboratory technician shall take out the materials and sampling utensils to be taken in the sampling room.
After sampling, the QC laboratory technician shall be responsible for the comprehensive cleaning of the sampling room.
The sampling vehicle is required to be thoroughly cleaned after each sampling. The method is: first wipe the inside and outside with a clean rag, and then wipe each part repeatedly with a clean special rag dipped in 75% ethanol several times.

(Figure 3: Clean room environment)
After sampling, the sampled materials shall be sealed in time, affixed with sampling certificates, and placed in the logistics room from the buffer room, and taken out and returned to the original location by the warehouse keeper. When the sampled materials enter the sampling room, the warehouse keeper shall conduct a comprehensive cleaning of the outer packaging before entering the buffer room for ultraviolet sterilization.
4.8 Non-sampling personnel and cleaning personnel shall not enter the sampling room at will.
4.9 The sampling vehicle shall conduct daily dust particle and sedimentation bacteria monitoring in accordance with the 10,000-level clean area.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


