Why Cleanrooms Are Non-Negotiable in Biopharmaceutical Manufacturing
Cleanrooms prevent life-threatening contamination in biopharma production. FDA requires ISO Class 5 environments (≤3,520 particles/m³ ≥0.5μm) for sterile processes like vaccine filling. HEPA/ULPA filtration achieves 99.99% particle retention, while unidirectional airflow maintains precise velocities.

Critical technologies ensure compliance: Isolators/RABS reduce human contamination by 68%, while real-time particle counters trigger alerts within 6 seconds. VHP bio-decontamination delivers 6-log microbial kill between batches.
These controls directly enable patient-safe therapies, meeting USP <797> and EU GMP Annex 1 sterility standards (SAL ≤10⁻⁶). Non-compliance risks product recalls and regulatory action.
Core Design Requirements for Compliance
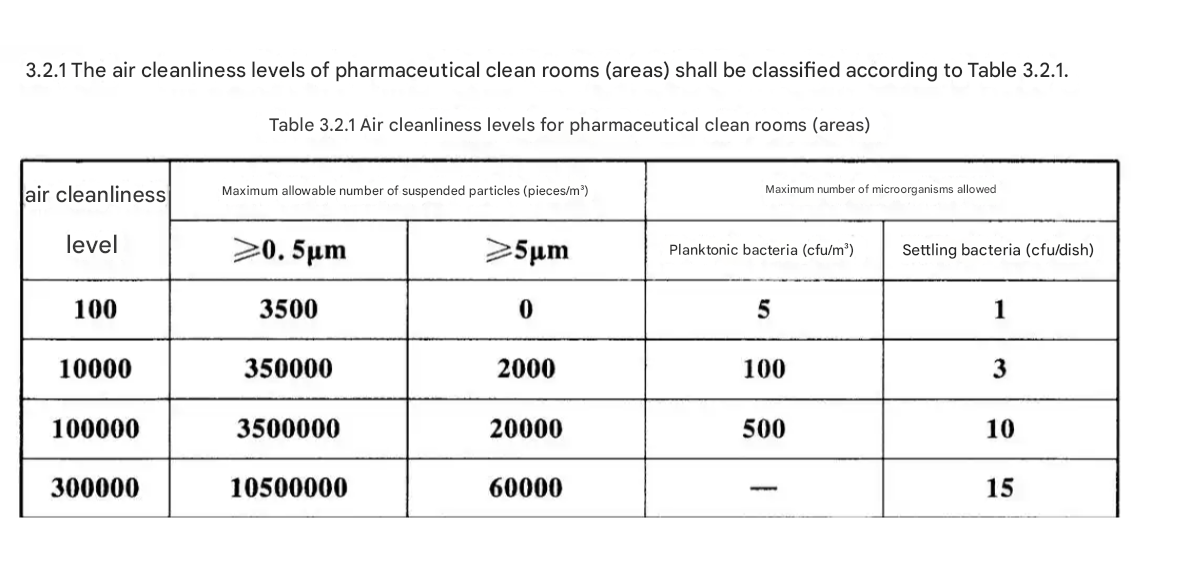
ISO 14644 classifications form the non-negotiable foundation of Cleanroom design, dictating particle limits for each cleanroom class. Biopharmaceutical facilities typically require ISO Class 5 (Grade A) environments for critical operations, mandating ≤3,520 particles ≥0.5μm per cubic meter.
Airflow & Pressure Control
Unidirectional vertical airflow (UDAF) must maintain 0.45 m/s±20% velocity in ISO 5 zones to prevent particle settlement. Pressure cascades with minimum 15 Pa differentials between adjacent rooms create directional airflow barriers, reducing cross-contamination risks by 92% according to FDA audit data.
Filtration Systems
HEPA/ULPA filters with 99.99% efficiency at 0.3μm are mandatory for all supply air. These critical components require annual integrity testing per ISO 14698 standards, with replacement triggered when pressure drop exceeds 50% of initial value.
Material & Surface Compliance
Non-shedding, cleanable materials must cover all surfaces without joints or crevices. Antimicrobial vinyl walls, conductive epoxy floors (resistance: 10⁶-10⁹ ohms), and coved corners prevent particle accumulation and meet EU GMP Annex 1 requirements.
| Parameter | ISO Class 5 | ISO Class 7 | Regulatory Reference |
|---|---|---|---|
| air changes/Hour | 240-480 | 30-40 | FDA Aseptic Processing |
| Temperature Control | 20-24°C ±1°C | 20-24°C ±2°C | USP <797> |
| Relative humidity | 45% ±5% | 45% ±10% | EU GMP Annex 1 |
monitoring & Validation
Continuous particle counting with ≤6-second alert response is required for Grade A zones. Automated monitoring systems must record temperature, humidity, and pressure differentials at 1-minute intervals, storing data for 1+ years per 21 CFR Part 11.
Compliance Tip: Integrate isolators or RABS (Restricted Access Barrier Systems) for high-risk operations. These provide physical separation from operators, reducing viable particle counts by 68% compared to open processing.
Mission-Critical Equipment & Technologies
Advanced CleanRoom technologies directly determine product sterility in biopharmaceutical manufacturing, with equipment selection impacting compliance with FDA 21 CFR Part 211 and EU GMP Annex 1 regulations.
Air Handling & Filtration Systems
HEPA/ULPA filters with 99.99% efficiency at 0.3μm form the foundation of contamination control. Integrated HVAC systems maintain ISO Class 5 conditions through ≥90 air changes/hour, with precise temperature (±1°C) and humidity (±5%) control critical for monoclonal antibody production. (See validation protocols)
Advanced Containment Solutions
Isolators and RABS (Restricted Access Barrier Systems) reduce human-borne contamination by 68% according to EMA studies. Closed-system isolators maintain O₂ levels<0.1% during lyophilization, while split-RABS designs enable faster changeovers for multi-product facilities. Glove integrity testing every shift prevents microbial ingress.
Real-Time Monitoring Systems
Laser particle counters detect deviations within 6 seconds at 1 CFM sampling rates. Modern systems integrate with EM (Environmental monitoring) software to map contamination risks, storing data per 21 CFR Part 11 requirements. Viable air samplers like impactors and centrifuges provide microbial counts within 4-hour incubation periods.
Filtration Efficiency
HEPA: 99.99% @ 0.3μm
ULPA: 99.999% @ 0.12μm
Containment Performance
Isolators: 6-log reduction
RABS: 4-log reduction
Monitoring Speed
Particle alerts: ≤6 sec
Data recording: 1-min intervals
Decontamination Technologies
Vaporized Hydrogen Peroxide (VHP) generators achieve 6-log spore reduction in 90-minute cycles, validated per ISO 14698 standards. Automated systems monitor concentration (200-1200 ppm) and humidity (35-85% RH) to ensure cycle efficacy, with residual detection sensitive to<1 ppm.
Operational Excellence & Maintenance Protocols
Cleaning Validation
Vaporized hydrogen peroxide (VHP) bio-decontamination cycles achieve 6-log reductions. Post-cycle residue testing must show<1 ppm residual peroxide – verified via ISO 14698 compliant methods.
Preventive Maintenance
HEPA filter integrity testing every 6 months prevents catastrophic failures. Use differential pressure monitors to track filter loading – replacing at ΔP >50% initial value maintains airflow stability.
Personnel Training
Gowning competency assessments reduce 68% of human-borne contamination. Video-based smoke studies prove proper technique prevents turbulent flows near critical zones.
Future-Proofing Your Facility
Single-use technologies (SUBs) and closed-system transfers eliminate cleaning validation for 80% of new facilities. Modular cleanrooms with soft-wall isolators now enable reconfiguration in 48 hours versus traditional 6-week renovations.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU



