
Introduction
Proper Pharmaceutical Cleanroom HVAC Design is critical for maintaining product quality and patient safety in pharmaceutical manufacturing. The HVAC system serves as the lungs of the cleanroom, controlling contamination, temperature, humidity, and pressure differentials that directly impact product quality.
Effective Pharmaceutical Cleanroom HVAC Design requires balancing multiple competing requirements including stringent contamination control, precise environmental parameters, energy efficiency, and regulatory compliance. At Deiiang™, our approach to Pharmaceutical Cleanroom HVAC Design focuses on creating systems that meet both current and future manufacturing needs while optimizing operational costs.
Key Challenges in Pharmaceutical Cleanroom HVAC Design:
- Maintaining iso 5 to iso 8 cleanliness classifications
- Precise temperature control (±1°C tolerance)
- Relative humidity control (±5% RH tolerance)
- Pressure cascade maintenance (10-15 Pa differentials)
- Energy consumption optimization
- Regulatory compliance (FDA, EMA, WHO)
- Cross-contamination prevention
- Validation and documentation requirements
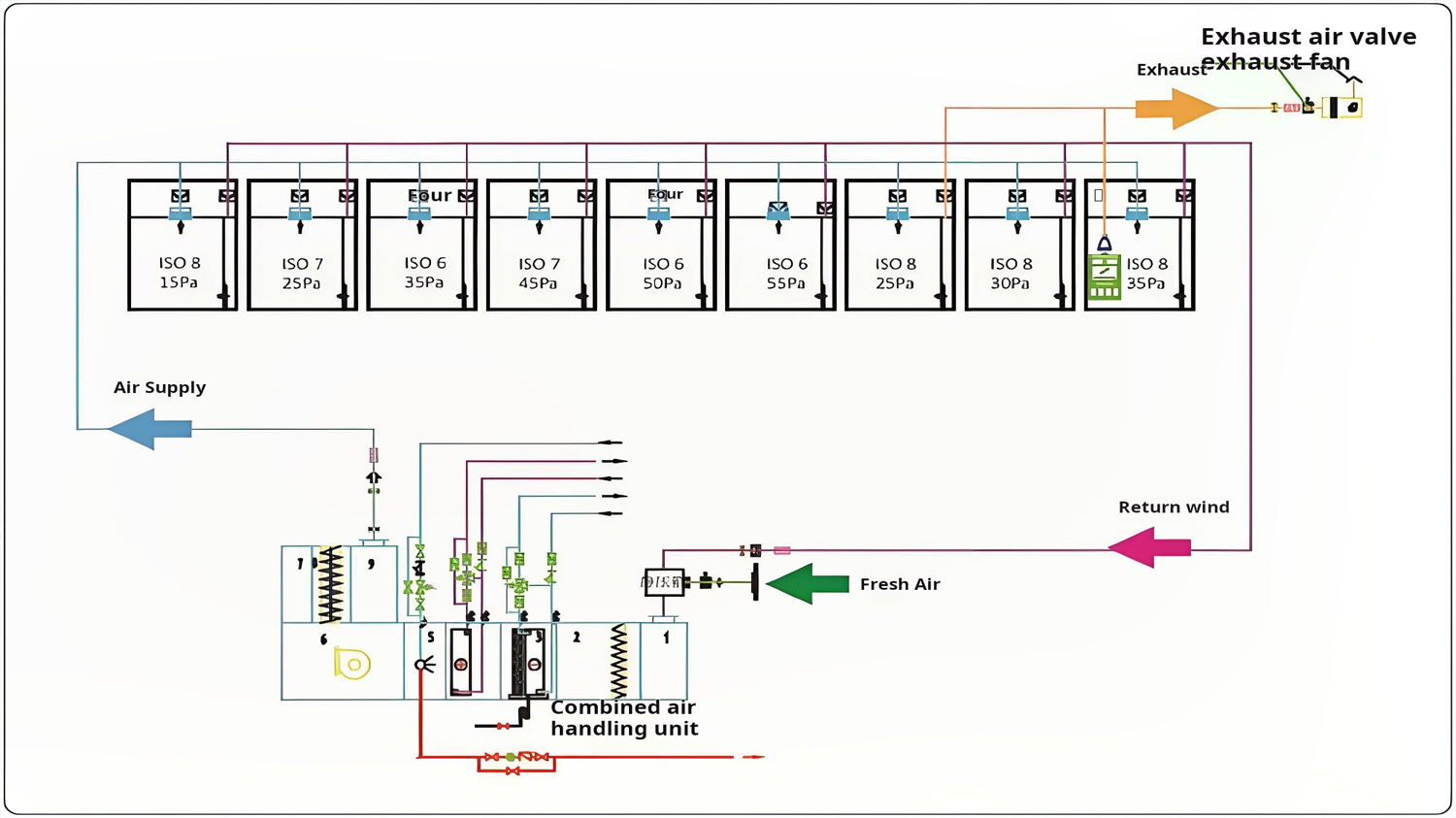
Figure 1: Typical Pharmaceutical Cleanroom HVAC System Layout
What is Pharmaceutical Cleanroom HVAC System? Basic Concepts and Components
A Pharmaceutical Cleanroom HVAC Design differs significantly from conventional HVAC systems through its enhanced filtration capabilities, precise environmental control, and specialized air distribution patterns. Unlike standard systems that focus primarily on comfort, Pharmaceutical Cleanroom HVAC Design prioritizes contamination control and process requirements.
The fundamental difference lies in the air quality standards - while a typical office HVAC might target 500,000 particles per cubic foot, a Grade A CleanRoom requires fewer than 3,520 particles ≥0.5μm per cubic meter. This level of cleanliness requires specialized Pharmaceutical Cleanroom HVAC Design approaches including HEPA filtration, unidirectional airflow, and sophisticated control systems.
HVAC System Airflow Process
1. Air Intake & Pre-filtration
2. Heating/Cooling Coils
3. Humidification/Dehumidification
4. Fan Pressure Boost
5. HEPA/ULPA Filtration
6. Cleanroom Supply
7. Return Air & Exhaust
Core Components
- Air Handling Units (AHU): Specially designed for pharmaceutical applications with stainless steel construction
- HEPA/ULPA Filters: 99.97% efficiency for 0.3μm particles (HEPA) or 99.999% for 0.12μm particles (ULPA)
- Ductwork: Antimicrobial coated or stainless steel with smooth internal surfaces
- Control Systems: DDC/BMS with data logging and alarm management
- Differential Pressure Controls: Maintain pressure cascades between zones
- Environmental monitoring: Continuous particle, temperature, humidity monitoring
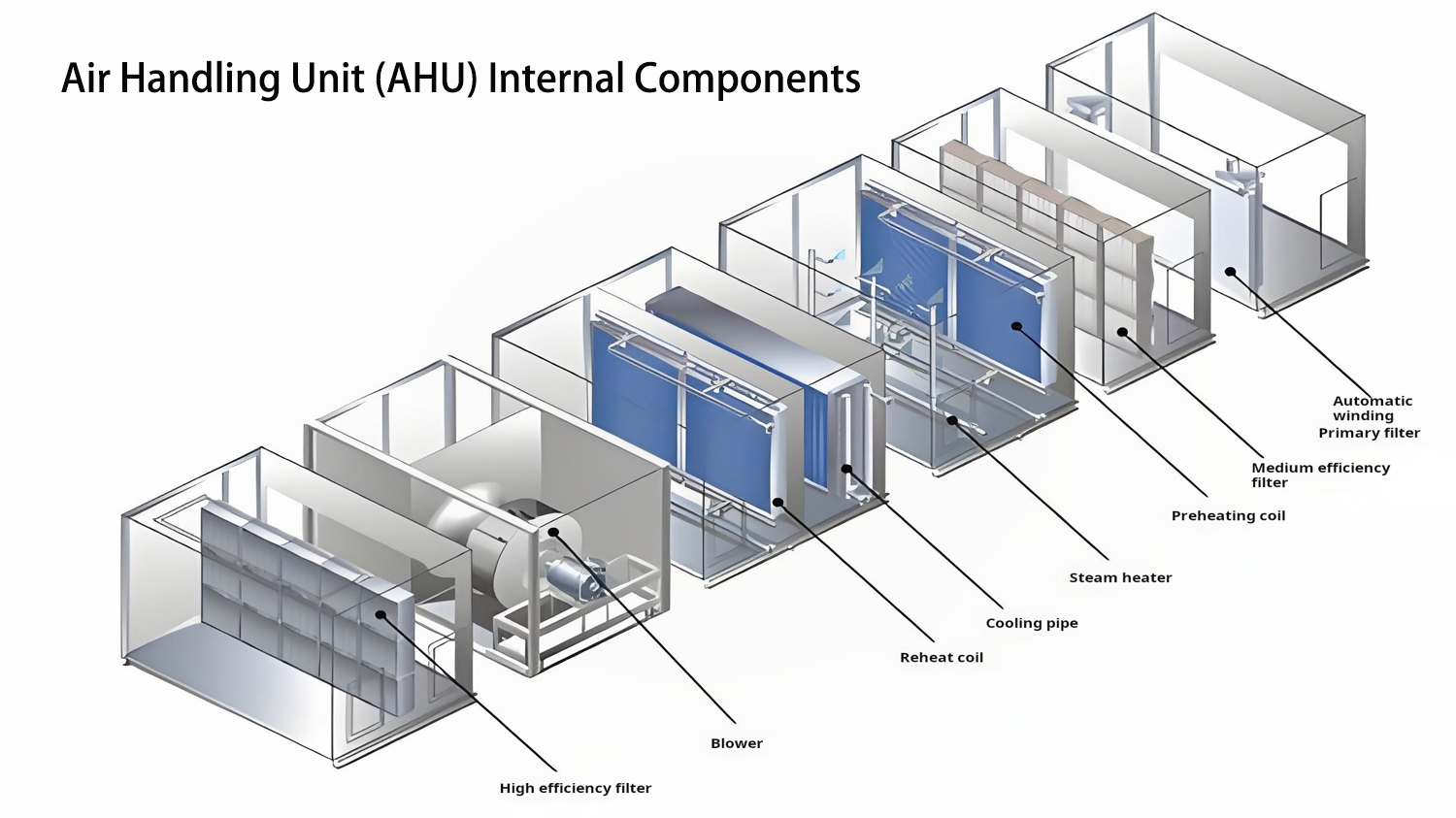
Figure 2: Air Handling Unit (AHU) Internal Components
Pharmaceutical CleanRoom Classification Standards and Regulatory Requirements
Understanding regulatory requirements is fundamental to successful Pharmaceutical Cleanroom HVAC Design. Global regulations establish clear standards for environmental conditions that directly impact HVAC system specifications. Proper Pharmaceutical Cleanroom HVAC Design must comply with multiple regulatory frameworks simultaneously.
At Deiiang™, our Pharmaceutical Cleanroom HVAC Design methodology incorporates regulatory requirements from the initial concept stage, ensuring compliance with FDA, EMA, and other relevant authorities. Our approach to Pharmaceutical Cleanroom HVAC Design focuses on creating systems that not only meet current regulations but are adaptable to evolving standards.
| ISO Class | gmp grade | Max Particles/m³ (≥0.5μm) | Recommended ACH | Typical Applications |
|---|---|---|---|---|
| ISO 5 | A | 3,520 | 240-600* | Aseptic filling, critical zones |
| ISO 6 | B | 35,200 | 90-120 | Background for Grade A zones |
| ISO 7 | C | 352,000 | 30-60 | Preparation areas, less critical zones |
| ISO 8 | D | 3,520,000 | 15-25 | Changing rooms, corridor areas |
*ISO 5 typically uses unidirectional airflow with velocity 0.45 m/s ±20% rather than ACH
Key Regulatory Requirements for Pharmaceutical Cleanroom HVAC Design:
- FDA cGMP (21 CFR Part 211): Requires adequate ventilation, air filtration, and pressure differentials
- EU GMP Annex 1: Detailed requirements for sterile medicinal products including HVAC specifications
- WHO GMP: Standards for pharmaceutical products with focus on contamination control
- PIC/S Guidelines: Harmonized GMP Standards across participating authorities
- ISO 14644 Series: Cleanroom standards for design, testing, and operation
Core Design Principles for Pharmaceutical Cleanroom HVAC Systems
The foundation of effective Pharmaceutical Cleanroom HVAC Design lies in understanding and properly implementing core design principles. These principles ensure the HVAC system can maintain the required environmental conditions consistently. Every aspect of Pharmaceutical Cleanroom HVAC Design must be carefully considered to achieve regulatory compliance and operational efficiency.
At Deiiang™, our Pharmaceutical Cleanroom HVAC Design process begins with comprehensive risk assessment and process understanding. We apply these design principles to create robust systems that maintain product quality while optimizing energy consumption and operational costs.
Airflow Patterns
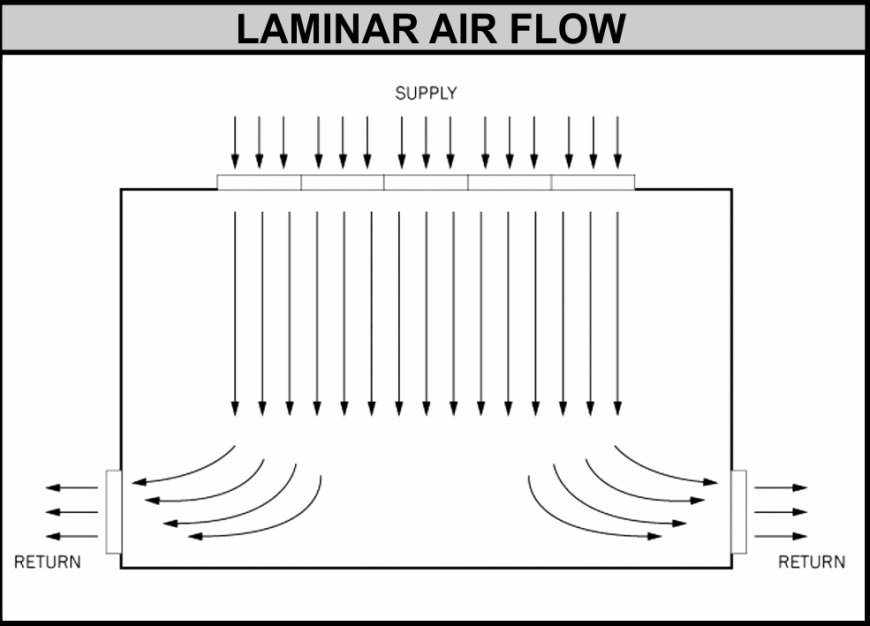
Unidirectional (Laminar) Airflow
Used in Grade A zones with consistent velocity (0.45 m/s ±20%) to protect critical processes from contamination.
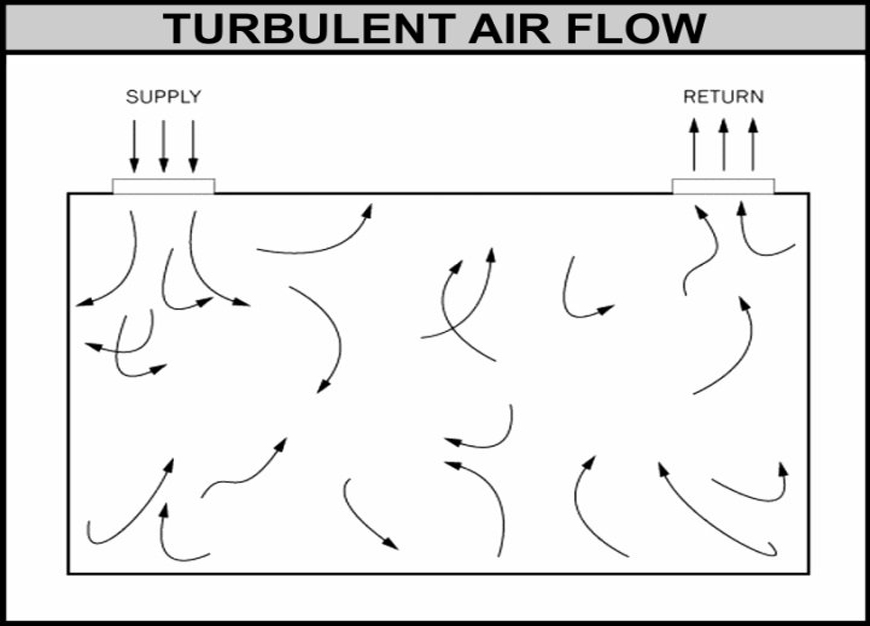
Turbulent (Non-unidirectional) Airflow
Used in Grades B, C, and D areas with sufficient air changes to dilute and remove contaminants.
Key Design Parameters
Pressure Differential Control
Maintains directional airflow from clean to less clean areas. Typical requirements:
- Grade A to Grade B: 10-15 Pa
- Grade B to grade c: 10-15 Pa
- Grade C to Grade D: 10-15 Pa
- Clean to non-clean: 15 Pa minimum
Temperature and Humidity Control
Typical requirements for comfort and process control:
- Temperature: 20-24°C ±1-2°C
- Relative Humidity: 45-55% ±5%
- More stringent controls for hygroscopic materials or specific processes
Air Change Rate Calculation
air changes per hour (ACH) = (Total Air Supply in m³/h) / (Room Volume in m³)
Example: For a 100m³ room with 3,000 m³/h supply: ACH = 3,000/100 = 30 ACH
Filtration System Design
Prefilters (G4-F7)
Capture larger particles (5-10μm) to protect HEPA filters and extend their lifespan. Efficiency: 35-80% for 0.4μm particles.
HEPA Filters (H13-H14)
Critical for final air cleaning. H13 efficiency: 99.95% for 0.3μm particles. H14 efficiency: 99.995% for 0.3μm particles.
ULPA Filters (U15-U17)
For ultra-clean applications. U15 efficiency: 99.9995% for 0.12μm particles. Used when higher cleanliness is required.
Pharmaceutical Cleanroom HVAC System Equipment Selection and Configuration
Proper equipment selection is crucial for successful Pharmaceutical Cleanroom HVAC Design. Each component must be carefully specified to meet the stringent requirements of pharmaceutical manufacturing environments. The right Pharmaceutical Cleanroom HVAC Design equipment ensures reliability, compliance, and optimal performance.
| Component | Key Selection Criteria | Deiiang™ Recommendation |
|---|---|---|
| Air Handling Unit | Stainless steel construction, double-wall panels, leak-tight design | Modular design with easy access for maintenance and cleaning |
| Fans | EC motors with VFD, redundant configuration for critical areas | Backward curved centrifugal fans with 15-20% spare capacity |
| Cooling Coils | Copper tubes with aluminum fins, antimicrobial coating | Staged cooling capacity for better humidity control |
| Heating Coils | Stainless steel construction, low fin density for cleanability | Electric or hot water coils with modulating control |
| Humidification | Pure steam injection or electrode boilers with clean steam | Clean steam humidifiers for sterile applications |
| Ductwork | Stainless steel 316L, welded seams, smooth internal surfaces | GMP-compliant installation with proper slope for drainage |
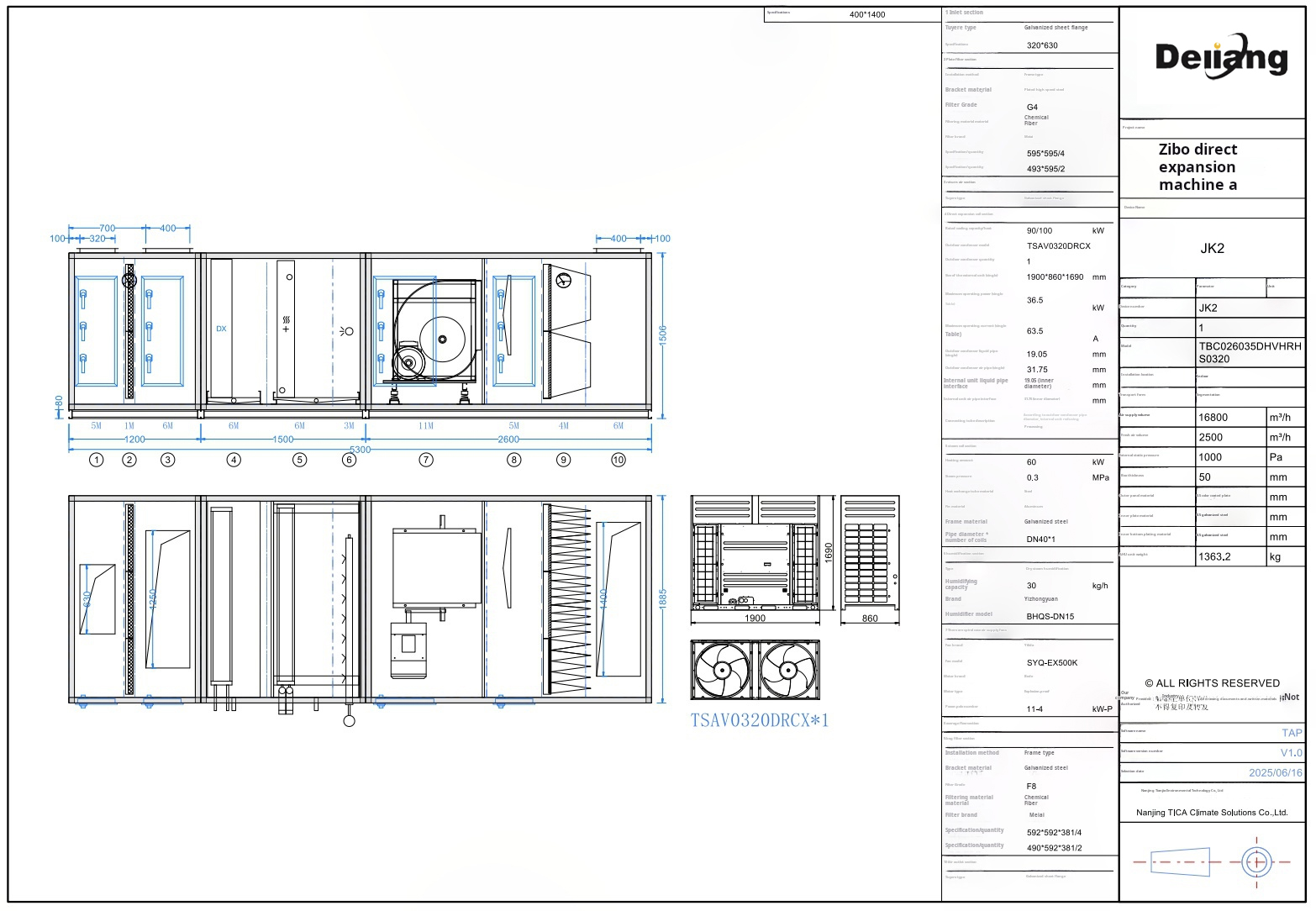
Figure 3: Typical Air Handling Unit Configuration for Pharmaceutical Applications
Critical Considerations for Pharmaceutical Cleanroom HVAC Design Equipment:
- Material Compatibility: All wetted surfaces must be compatible with cleaning and sanitizing agents
- Cleanability: Smooth surfaces, minimal ledges, proper drainage slopes
- Containment: Appropriate sealing for aseptic and containment applications
- Redundancy: Backup systems for critical components in Grade A and B areas
- Documentation: Complete traceability and validation documentation package
Energy Optimization and Sustainable Design
Pharmaceutical Cleanroom HVAC Design typically accounts for 60-70% of a facility's total energy consumption, making energy efficiency a critical consideration. Optimizing Pharmaceutical Cleanroom HVAC Design for energy performance can reduce operational costs by 30-50% while maintaining compliance.
Modern Pharmaceutical Cleanroom HVAC Design incorporates multiple energy-saving strategies without compromising environmental control. At Deiiang™, our Pharmaceutical Cleanroom HVAC Design approach balances energy efficiency with regulatory requirements, creating sustainable solutions that reduce both environmental impact and operating costs.
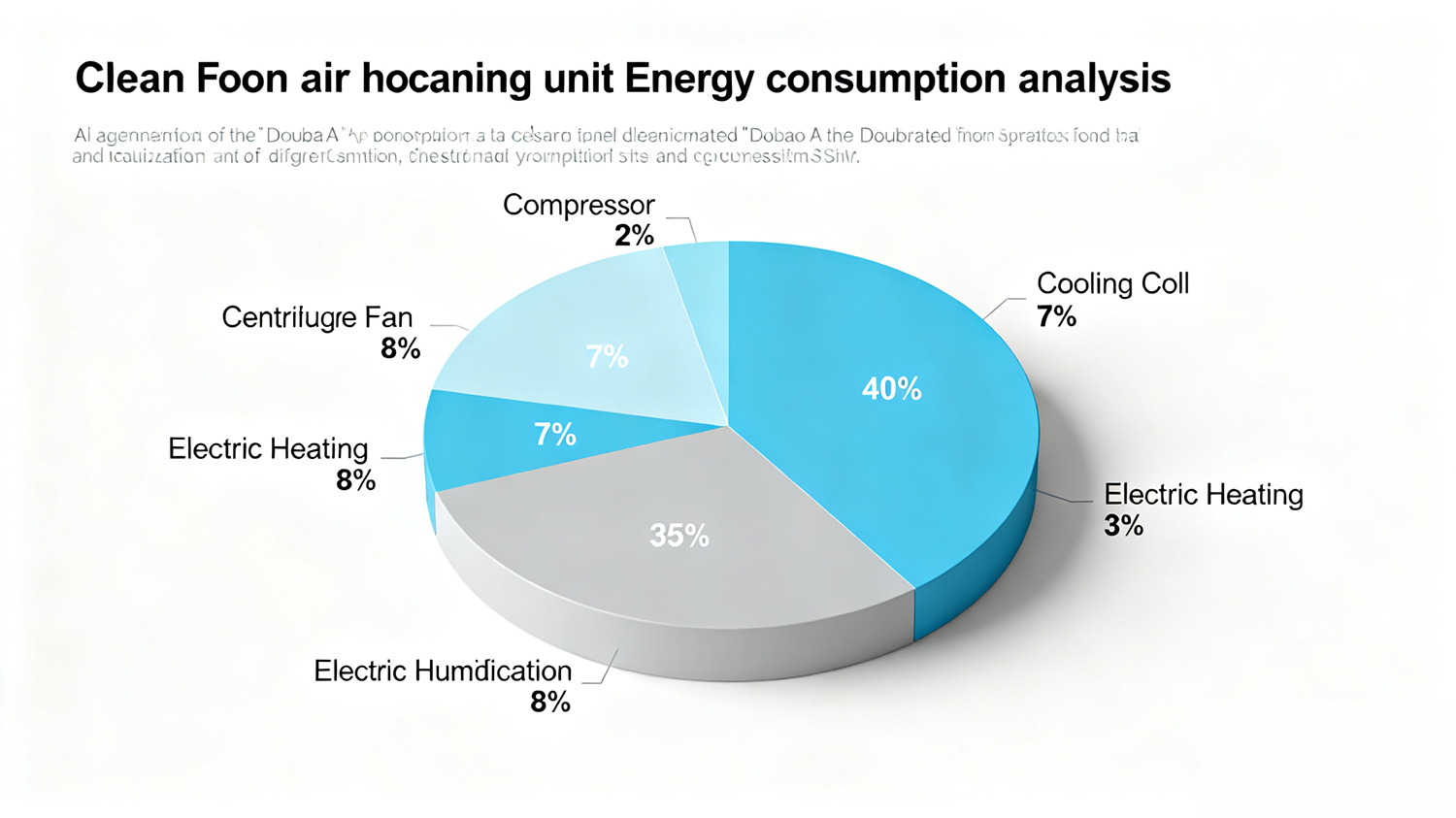
Figure 4: Typical Energy Consumption Breakdown for Pharmaceutical Cleanroom HVAC Systems
Energy Efficiency Strategies for Pharmaceutical Cleanroom HVAC Design:
Variable Frequency Drives (VFD)
Reduce fan energy by 30-50% by matching airflow to actual demand rather than running at constant volume.
Heat Recovery Systems
Recover 50-80% of exhaust air energy using run-around coils, heat pipes, or thermal wheels.
Demand-Based Control
Reduce ACH during unoccupied periods while maintaining pressure differentials and cleanliness.
High-Efficiency Motors
Premium efficiency (IE4/IE5) motors reduce energy consumption by 2-8% compared to standard motors.
Optimized ACH Rates
Right-sizing ACH based on actual contamination risk rather than conservative estimates.
Free Cooling
Use outdoor air for cooling when ambient conditions permit, reducing mechanical cooling load.
Case Study: Energy Optimization in Pharmaceutical Cleanroom HVAC Design
Client: Major pharmaceutical manufacturer with 500m² ISO 7 Cleanroom
Challenge: High energy costs ($120,000 annually) and frequent filter changes
Deiiang™ Solution:
- Implemented VFDs on supply and return fans (28% energy reduction)
- Installed run-around coil heat recovery (42% thermal energy recovery)
- Optimized ACH from 45 to 35 based on risk assessment
- Upgraded to lower pressure drop HEPA filters
Results: 38% reduction in energy costs ($45,600 annual savings), 25% longer filter life, and maintained ISO 7 classification with improved stability.
Commissioning, Qualification and Validation (CQV)
Comprehensive validation is essential for Pharmaceutical Cleanroom HVAC Design to demonstrate that the system consistently performs as intended. The validation process follows a structured approach from design through operational qualification.
Proper Pharmaceutical Cleanroom HVAC Design validation provides documented evidence that the system meets user requirements and regulatory standards. At Deiiang™, our validation approach for Pharmaceutical Cleanroom HVAC Design ensures complete traceability and compliance throughout the system lifecycle.
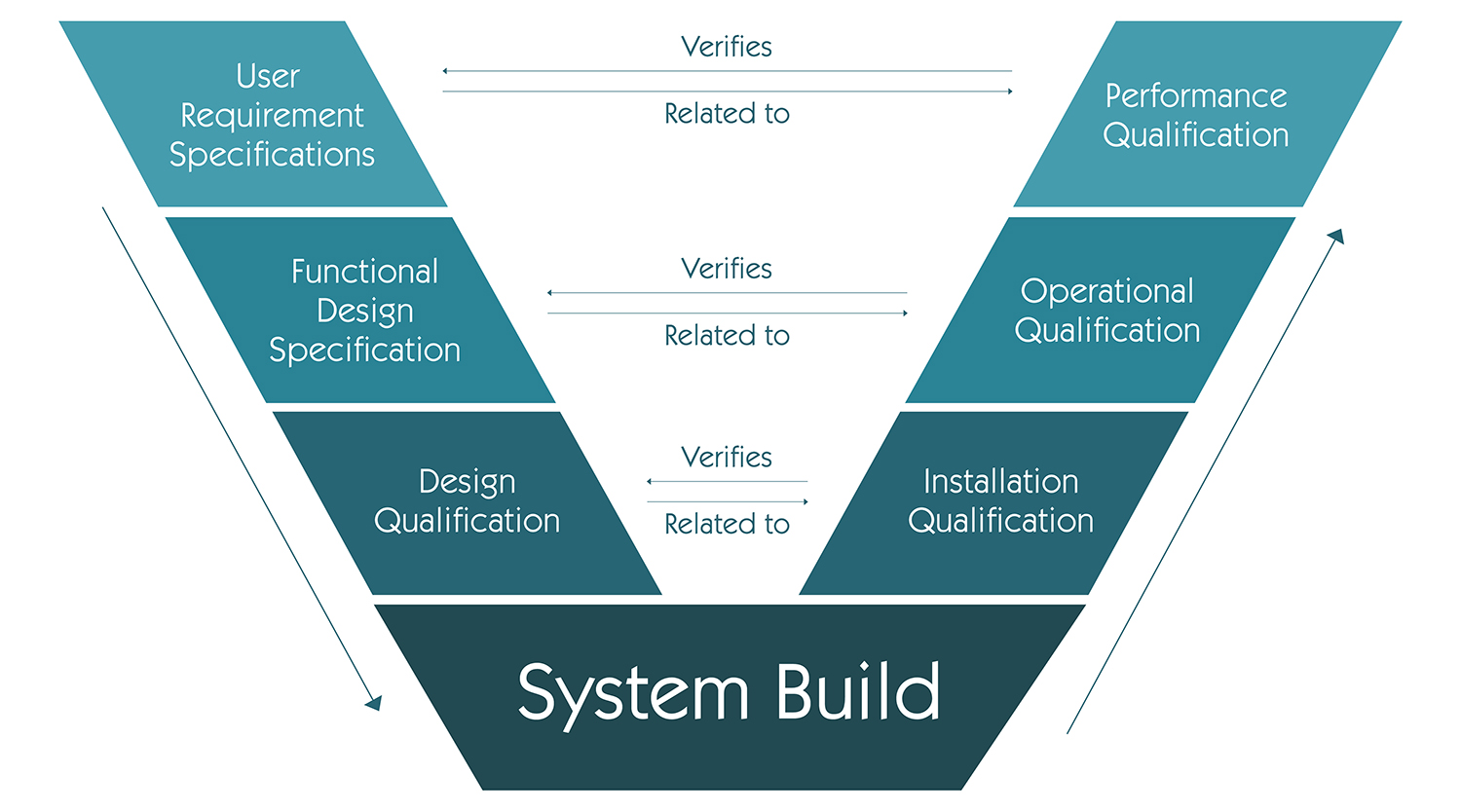
Figure 5: HVAC System Validation V-Model
| Validation Phase | Key Activities | Deliverables |
|---|---|---|
| Design Qualification (DQ) | Verify design meets URS and regulatory requirements | DQ Protocol and Report |
| Installation Qualification (IQ) | Verify proper installation per design specifications | IQ Protocol and Report |
| Operational Qualification (OQ) | Verify system operates as intended under all operational ranges | OQ Protocol and Report |
| Performance Qualification (PQ) | Verify consistent performance under routine operating conditions | PQ Protocol and Report |
Critical Testing Requirements for Pharmaceutical Cleanroom HVAC Design Validation:
HEPA Filter Integrity Testing
Performed per IEST-RP-CC034 using thermal or photometric methods to verify no leaks ≥0.01% of upstream concentration.
Airflow Velocity and Uniformity
Unidirectional airflow: 0.45 m/s ±20% measured 150-300mm from filter face.
Particle Counting
iso 14644-1 classification verification with minimum sample locations based on room area.
Pressure Differentials
Verify cascading pressure between zones (typically 10-15 Pa) with all doors closed and during door opening tests.
Recovery Testing
Measure time to recover from simulated contamination event (typically ≤15-20 minutes for ISO 7).
Temperature and Humidity Mapping
Verify uniform distribution within specified ranges (typically 20-24°C ±2°C, 45-55% ±5% RH).
Operation, Maintenance and Risk Management
Effective operation and maintenance are critical for sustaining Pharmaceutical Cleanroom HVAC Design performance throughout the system lifecycle. A comprehensive maintenance program ensures continuous compliance and prevents costly downtime.
| Common Issue | Potential Causes | Recommended Actions |
|---|---|---|
| Loss of Pressure Differential | Door left open, filter clogging, fan issues, control system fault | Check door status, inspect filters, verify control setpoints, calibrate sensors |
| High Particle Counts | HEPA filter damage, improper gowning, excessive personnel activity | Perform HEPA integrity test, review procedures, retrain personnel |
| Temperature/Humidity Excursions | Sensor drift, coil fouling, steam supply issues, control problems | Calibrate sensors, clean coils, check steam system, review control logic |
| Increased Energy Consumption | Filter loading, improper control sequences, mechanical issues | Replace filters, optimize control sequences, perform energy audit |
Preventive Maintenance Checklist
- Daily: Visual inspection, alarm review, parameter verification
- Weekly: Filter pressure drop check, sensor verification
- Monthly: Control system backup, trending analysis
- Quarterly: Calibration verification, belt tension check
- Annually: HEPA integrity testing, comprehensive calibration
- As Needed: Filter replacement based on pressure drop
Filter Replacement Guidelines
HEPA filters should be replaced when:
- Final pressure drop reaches 1.5x initial clean pressure drop
- Integrity test failure cannot be repaired
- Physical damage is observed
- After 10 years of service (recommended maximum)
Prefilters should be replaced at 70-80% of terminal pressure drop to protect HEPA filters.
Conclusion and Call to Action
Proper Pharmaceutical Cleanroom HVAC Design is fundamental to pharmaceutical manufacturing quality, compliance, and efficiency. From regulatory requirements to energy optimization, each aspect requires specialized expertise and careful consideration.
At Deiiang™, we combine technical expertise with practical experience to deliver Pharmaceutical Cleanroom HVAC Design solutions that meet your specific needs. Our approach ensures regulatory compliance, operational efficiency, and long-term reliability.
Need assistance with your Pharmaceutical Cleanroom HVAC Design project? Contact Deiiang™ today for a consultation with our experts, including product designer Jason.peng.
 +86 18186671616
+86 18186671616 Jason@cleanroomequips.com
Jason@cleanroomequips.com
 MENU
MENU


